Hepatitis E virus (HEV) infection is one of the major public health problems in developing countries. HEV can cause chronic infections in immunocompromised individuals e.g. thalassemic patients with increased risk of morbidity and mortality. In addition there is possibility of HEV transmission through blood transfusion. Therefore, the present study aimed to investigate the seroprevalence and risk factors of HEV infection in β-thalassemic children.
MethodsThis cross-sectional study was conducted on 140 Egyptian children suffering from β-thalassemia, attending the hematology outpatient clinic from April to October 2016. Serum samples from patients were collected and anti-HEV antibodies; Immunoglobulin G (IgG) and Immunoglobulin M (IgM)
were measured by enzyme-linked immunosorbent assay (ELISA).
ResultsThe seroprevalence of HEV in β-thalassemic chidren was relatively high (27.15%). Anti-HEV IgG prevalence was 24.29% while that of IgM was 2.86%. There was significant association between HEV infection and age, residence, liver enzymes and amount of blood transfusion per year.
ConclusionsThalasemic patients are vulnerable to chronicity and increased risk of morbidity and mortality from HEV infection. Frequent assessment of liver enzymes in thalassemic patients to monitor subclinical HEV is recommended. Close monitoring and HEV screening of blood donations should be taken in consideration. Public awareness about HEV endemicity, modes of transmission, and risk hazards especially in high risk group should be done to reduce the disease burden.
Hepatitis E virus infection is endemic in many developing countries while sporadic HEV infections have also been reported in some developed countries.1 Although HEV infection is usually associated with acute self-limited hepatitis, fulminant hepatic failure with morbidity and mortality may occur especially in immunocompromised hosts.2 High prevalence of HEV infection was reported in Africa, Central and Southeast Asia, and Mexico.3 In France, the seroprevalence of HEV (anti-HEV immunoglobulin G (IgG) and IgM) varied from 21.9% to 71.3%, according to geographic distribution, with average 39.1%.4 People with low educational level may be at a higher risk of exposure and infection than those with higher educational level. In Egypt, low educational level and socioeconomic status resulted in lack of knowledge about avoidable possible risk factors associated with HEV infection. Thus HEV was highly endemic in Egyptian rural communities with genotype 1 subtype 3 most circulating.5,6 There are four documented routes of transmission of HEV including fecal-oral route, vehicle-borne including water-borne and zoonotic foodborne, bloodborne transmission through parenteral blood transfusion, and perinatal via vertical transmission from mother-to-infant.7,8 Chronic HEV infection was acquired in 1.4% of patients following liver transplantion which subsequently resulted in persistent damage of the liver graft.9 Thalassemia is one of the most common genetic diseases worldwide. It is a major public health problem, causing much morbidity, early mortality, and great financial and emotional burden for a family. Thalassemia is the most common form of inherited anemia around the world.10 β-thalassemia frequencies are common in almost all Arab countries.11 In Egypt, β-thalassemia is the most common type with a carrier rate ranging from 5.3% to ≥9%. It was estimated that 1000/1.5 million live births/year suffer from thalassemia disease in Egypt.12 Many thalassemia patients develop chronic liver disease (CLD) like chronic hepatitis C. HAV and HEV infections show more severe clinical courses in the CLD patients.13 Some authors documented HEV seropositivity among hemodialysis and thalassemia patients.14,15 It was reported that HEV RNA detected in blood donations through studies by blood banks in several European countries.16 Therefore, some countries began HEV RNA screening of blood donations and others selectively applied blood screening for high risk patients.17 A previous study conducted on patients with acute viral hepatitis in Egypt reported HEV infection in about 12–42% of all cases.18 Another study carried out on blood samples collected from 134 jaundiced patients in Egypt, revealed that 51 (38.1%) patients were positive for anti-HEV IgG.19 Also, study on children with acute viral hepatitis in Assiut, Egypt, detected 30.9% HEV infection in children.20 The present study aimed to detect the seroprevalence of HEV infection in thalassemic children to assess the role of blood transfusion as a risk factor for HEV infection.
Patients and methodsPatientsThis cross-sectional study was conducted on 140 Egyptian children suffering from β-thalassemia, attending the hematology outpatient clinic in Mansoura University Children's Hospital (MUCH) from April 2016 through October 2016. Patients included in this study were patients with beta-thalassemia aged up to 18 years who frequently received blood transfusion. However, patients with hemolytic anemia other than thalassemia, patients with alpha-thalassemia, and patients with less than 10 blood transfusions were excluded from the study. This study was approved by the Medical Research Ethics Committee, Mansoura University (IRB no. MS/16/03/82).
Data collected from patientsData collected from patients included age, sex, residence, amount of blood transfused per year, abdominal examination of liver and spleen, serum ferritin, liver function tests (ALT and AST), and viral hepatitis markers (HBs Ag and HCV Abs).
Sample collection and processingTwo milliliters of venous blood were obtained from each selected patient and collected in a sterile tube, centrifuged immediately for serum separation. Serum samples were stored at −20°C. Presence of anti-HEV antibodies (anti-HEV IgG indicating past infection and anti-HEV IgM indicating recent infection) in the patients’ sera were measured by enzyme-linked immunosorbent assay; HEV-IGM Elisa (Immunospec, USA, BL147710) and HEV-IGG Elisa (Immunospec, USA, BL147709).
Statistical analysisThe collected data was coded, processed and analyzed using SPSS (Statistical package for social science) version 23 for windows. Descriptive statistics were calculated by frequency (number-percent). Chi square test was used for inter-group comparison of categorical data. p-Value less than 0.05 (5%) was considered statistically significant. Significant variables on univariate analysis were entered into a logistic regression model to determine which of these factors were independently associated with HEV infection, followed by multivariate analysis.
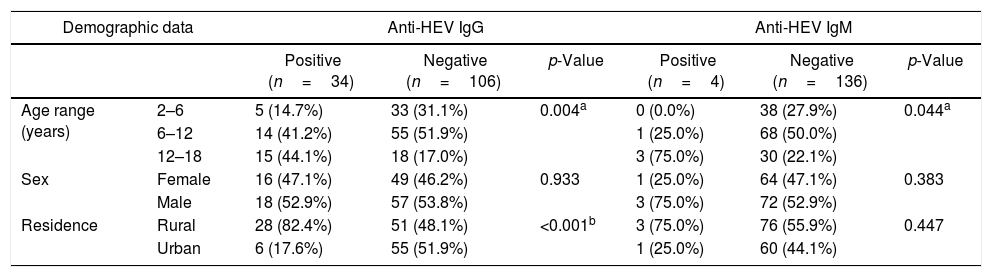
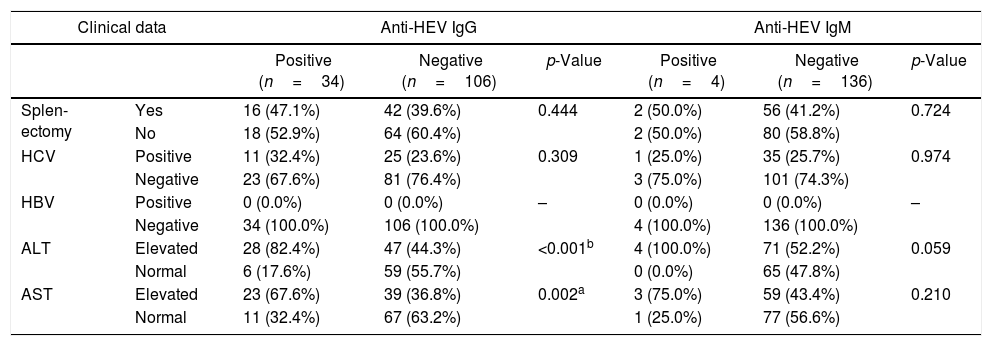
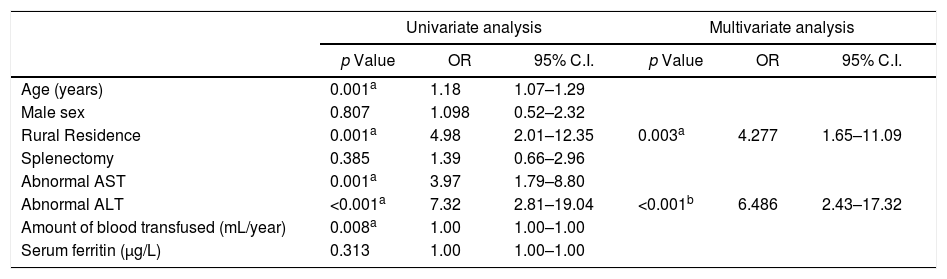
ResultsThe seroprevalence of HEV antibodies in β-thalassemic patients was 27.15% (38/140 patients); 34 (24.29%) patients were positive for anti-HEV IgG, and four (2.86%) patients were positive for anti-HEV IgM. As shown in Table 1, there was significant association between age and anti-HEV IgG and IgM seropositivity (p=0.004, and 0.044, respectively). HEV seropositivity increased with age. There was significant association between rural residence and anti-HEV IgG (p<0.001). Regarding clinical data, there was significant association between liver enzymes and anti-HEV IgG. No patients in our study had HBV infection. Splenectomy and HCV infection were not significantly associated with HEV seropositivity (Table 2). Table 3. In Univariate analysis, there was significant association between Age, residence, liver enzymes, and amount of blood transfusions per with HEV seropositivity. On multivariate analysis only rural residence and elevated ALT levels were independently associated with HEV seropositivity.
Demographic data associated with HEV seropositivity in thalassemic children.
| Demographic data | Anti-HEV IgG | Anti-HEV IgM | |||||
|---|---|---|---|---|---|---|---|
| Positive (n=34) | Negative (n=106) | p-Value | Positive (n=4) | Negative (n=136) | p-Value | ||
| Age range (years) | 2–6 | 5 (14.7%) | 33 (31.1%) | 0.004a | 0 (0.0%) | 38 (27.9%) | 0.044a |
| 6–12 | 14 (41.2%) | 55 (51.9%) | 1 (25.0%) | 68 (50.0%) | |||
| 12–18 | 15 (44.1%) | 18 (17.0%) | 3 (75.0%) | 30 (22.1%) | |||
| Sex | Female | 16 (47.1%) | 49 (46.2%) | 0.933 | 1 (25.0%) | 64 (47.1%) | 0.383 |
| Male | 18 (52.9%) | 57 (53.8%) | 3 (75.0%) | 72 (52.9%) | |||
| Residence | Rural | 28 (82.4%) | 51 (48.1%) | <0.001b | 3 (75.0%) | 76 (55.9%) | 0.447 |
| Urban | 6 (17.6%) | 55 (51.9%) | 1 (25.0%) | 60 (44.1%) | |||
n, number.
Clinical parameters associated with HEV seropositivity in thalessemic children.
| Clinical data | Anti-HEV IgG | Anti-HEV IgM | |||||
|---|---|---|---|---|---|---|---|
| Positive (n=34) | Negative (n=106) | p-Value | Positive (n=4) | Negative (n=136) | p-Value | ||
| Splen-ectomy | Yes | 16 (47.1%) | 42 (39.6%) | 0.444 | 2 (50.0%) | 56 (41.2%) | 0.724 |
| No | 18 (52.9%) | 64 (60.4%) | 2 (50.0%) | 80 (58.8%) | |||
| HCV | Positive | 11 (32.4%) | 25 (23.6%) | 0.309 | 1 (25.0%) | 35 (25.7%) | 0.974 |
| Negative | 23 (67.6%) | 81 (76.4%) | 3 (75.0%) | 101 (74.3%) | |||
| HBV | Positive | 0 (0.0%) | 0 (0.0%) | – | 0 (0.0%) | 0 (0.0%) | – |
| Negative | 34 (100.0%) | 106 (100.0%) | 4 (100.0%) | 136 (100.0%) | |||
| ALT | Elevated | 28 (82.4%) | 47 (44.3%) | <0.001b | 4 (100.0%) | 71 (52.2%) | 0.059 |
| Normal | 6 (17.6%) | 59 (55.7%) | 0 (0.0%) | 65 (47.8%) | |||
| AST | Elevated | 23 (67.6%) | 39 (36.8%) | 0.002a | 3 (75.0%) | 59 (43.4%) | 0.210 |
| Normal | 11 (32.4%) | 67 (63.2%) | 1 (25.0%) | 77 (56.6%) | |||
ALT, alanine transaminase; AST, aspartate transaminase; n, number.
Univariate and multivariate analyses of factors associated with HEV seropositivity in thalassemic children.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| p Value | OR | 95% C.I. | p Value | OR | 95% C.I. | |
| Age (years) | 0.001a | 1.18 | 1.07–1.29 | |||
| Male sex | 0.807 | 1.098 | 0.52–2.32 | |||
| Rural Residence | 0.001a | 4.98 | 2.01–12.35 | 0.003a | 4.277 | 1.65–11.09 |
| Splenectomy | 0.385 | 1.39 | 0.66–2.96 | |||
| Abnormal AST | 0.001a | 3.97 | 1.79–8.80 | |||
| Abnormal ALT | <0.001a | 7.32 | 2.81–19.04 | <0.001b | 6.486 | 2.43–17.32 |
| Amount of blood transfused (mL/year) | 0.008a | 1.00 | 1.00–1.00 | |||
| Serum ferritin (μg/L) | 0.313 | 1.00 | 1.00–1.00 | |||
ALT, Alanine transaminase; AST, Aspartate transaminase; p, probability; OR, odd's ratio; CI, confidence interval.
The prevalence of HEV antibodies among thalassemic children in our study was 27.15%, 24.29% anti-HEV IgG positive and 2.86% anti-HEV IgM positive. Results of previous studies showed seroprevalence of HEV antibodies of 2.4% in thalassemic patients in Scandinavia21 and 10.7% in Saudi Arabia.22 In Iran, the seroprevalence of HEV IgG and HEV IgM were 10% and 1.8%, respectively.15 Reasons such as differences in socioeconomic, cultural, hygienic, and climatic factors across geographical areas may explain the lower prevalence rates in developed countries compared to developing countries.24 In our study, age was significantly associated with HEV antibodies. The highest HEV prevalence rate found in the age group 12–18 years indicated that the risk of HEV infection increased with age. Previous studies carried out in Egypt25 and in other geographic regions reported the same finding.26–28 This could be explained by the fact that older children had likely received more transfused blood, in addition to have been more exposed to contaminated junk food than younger children. In this study, the prevalence of HEV antibodies in rural areas (81.6%) was higher than in urban areas (18.4%). In rural Egypt, more than 60% of children with aged over 10 years had positive anti-HEV IgG.29 On the contrary, another study conducted on HCV infected thalassemic patients in Iran reported higher HEV seropositivity in thalassemic patients from urban regions than those from rural areas.6 Regarding liver enzymes, 84.2% of patients with HEV seropositivity showed elevated ALT levels, compared to 68.4% of elevated AST levels. Liver enzymes were higher among those with non-HEV antibodies compared to those with HEV antibodies. This may be due to presence of other causes of hepatitis either viral causes, mostly HCV, or non-viral causes. Although 100% and 75% of anti-HEV IgM positive patients had elevated ALT and AST levels, respectively, there was no significant association between liver enzymes and anti-HEV IgM seropositivity, probably due to small number of anti-HEV IgM positive patients. Previous studies reported that subclinical HEV infection might be the cause of elevated ALT.30 Therefore, in cases of unexplained ALT and AST elevation, HEV may be the reasonable cause. In two studies conducted in Japan on voluntary blood donors with elevated ALT levels, anti-HEV IgG was positive in 3.7% and 7.1% of participants.31,32 These findings suggest that patients with ongoing subclinical HEV infection had the potential to transmit HEV through blood transfusion. Our study revealed that there was significant association between the amount of blood transfused per year and HEV seropositivity (p=0.008). On the other hand, a previous study showed no significant association between the amount of blood transfused and anti-HEV seropositivity in thalassemic patients.21 This difference may be due to geographic difference and use of assays with low sensitivity that could underestimate HEV prevalence.33 Studies conducted in blood banks of several European countries reported that about 0.02–0.14% of blood donations were HEV RNA positive.16,34 This finding supports the possibility of HEV transmission through blood transfusion. Since recently in Ireland, blood donations are routinely screened for HEV RNA. Also in 2017, the UK, and Netherlands began HEV RNA screening in blood donations. In France and Germany, screening selectively occurs in high-risk patients, while blood authorities in Italy, Spain, Portugal and Greece are assessing whether HEV screening should be implemented or not.17 A previous study conducted in Egypt showed that about 0.45% of blood donors have ongoing subclinical HEV infection.35 It was reported that the risk of transmission through blood transfusion is increased in high-risk patients in developing regions than in other locations.23 HEV is endemic in Egypt and besides the possibility of HEV transmission through blood, our study showed significant association between amount of blood transfused and HEV seropositivity. Therefore, further studies should be carried out to detect HEV infection in risk group patients and blood donors.
ConclusionIn conclusion, the seroprevalence of HEV in β-thalassemic patients was relatively high. Therefore, close monitoring of these patients in addition to HEV screening of blood donations should be taken into consideration. Frequent assessment of liver enzymes in β-thalassemic patients to monitor subclinical HEV infections and other viral infections is recommended. Public awareness about HEV endemicity, modes of transmission, and risk hazards, especially in high-risk groups should be done to reduce the disease burden.
FundingThe authors received no specific funding for this work.
Conflicts of interestThe authors declare no conflicts of interest.