Patients HIV+ attending in a reference clinic, Southern Brazil.
ObjectiveTo compare the interferon-gamma-release assay (IGRA – QuantiFERON® TB Gold In-Tube) with the tuberculin skin test (TST – PPD-Rt 23) for latent tuberculosis infection (LTBI) in patients with HIV.
DesignCohort study. Patients were simultaneously submitted to the TST and blood collection for the IGRA.
ResultsA total of 140 subjects were included. Nine (6.4%) were IGRA+/TST+, 12 (8.6%) were IGRA+/TST−, 4 (3%) were IGRA−/TST+, and 115 (82%) IGRA−/TST−. There was poor agreement between tests (kappa=0.2), and no correlation between these results and CD4+ T lymphocyte counts. During follow-up, one patient with negative results on both tests died from sepsis, and another with discordant results (IGRA+/TST−) exhibited TST seroconversion. Compared to the TST, IGRA showed a sensitivity and specificity of 69% and 90%, respectively. The IGRA detected 8% more positive results than the TST. All patients were followed up for 2 years.
ConclusionThe higher accuracy of the IGRA would result in LTBI treatments being administered to patients who would have otherwise been overlooked, decreasing the number of active tuberculosis cases. The long-term survival of HIV carriers requires further evaluation.
In 2012, approximately 8.6 million people worldwide developed tuberculosis (TB),1–3 with the majority occurring in Asia (55%) and Africa (30%).4,5 Brazil is among the 22 countries with high TB burden; in 2012, 70,000 new cases of TB were detected in Brazil and approximately 4600 patients died of the disease. In Paraná State the incidence of tuberculosis is 22 for every 100,000 inhabitants. In addition, HIV/TB co-infection is the main cause of death with defined etiology among AIDS patients.4–6 Preliminary data from the Brazilian Ministry of Health show an average of 9.7% of HIV/TB co-infection; the rate is highest in the state of Rio Grande do Sul in Southern Brazil at approximately 19%.7,8
One of the main tasks of the Stop TB Partnership project, which aims to control the worldwide impact of TB, is more rapid detection and treatment of latent TB infection (LTBI), particularly in HIV-positive patients.5 Latent infection occurs through initial first contact with Mycobacterium tuberculosis and has no signs of active infection. At this stage, the tuberculin skin test (TST) can detect an immune response against the microorganisms that may persist throughout a person's lifetime.6 However, there is a risk of disease reactivation, which occurs in 2–23% of immunocompetent patients and at a rate of least 10% per year in immunocompromised individuals.6,9 LTBI treatment with isoniazid monotherapy can reduce the possibility of active disease progression and is recommended for patients with risk factors such as HIV and immunosuppressive drug treatment.10,11
The TST is a widely used immunodiagnostic test for LTBI detection.9 However, this technique has several disadvantages such as false-positive results in vaccinated individuals9,12–15; many lost readings14–16; false-negative results in individuals with recent active TB, malnutrition, congenital immunodeficiency, malignant neoplasms, disseminated TB, systemic viral diseases, live-virus vaccines, and immunosuppression (e.g., patients with HIV with CD4+ T lymphocyte counts <400cells/mm3)12,17; and errors in technical implementation and/or reading of the results.16,18,19
In vitro interferon-gamma (IFN-γ) release assays (IGRAs) are an alternative for detecting LTBI that have become a widespread mainly in developed countries, which have low TB prevalence.9,20,21 IGRAs are based on measuring IFN-γ released from CD4+ T lymphocytes upon stimulation by M. tuberculosis-specific antigens.21 Two commercial IGRAs are currently available: QuantiFERON® TB Gold In-Tube (Cellestis Limited, Victoria, Australia), which is based on an enzyme immunoassay, and the T-SPOT®.TB test (T-Spot; Oxford Immunotec, Abingdon, UK), which detects the numbers of IFN-γ-producing cells represented as spot-forming units.22 IFN-γ release indicates the presence of TB infection either latent or disease.2 Unlike the TST, neither Bacillus Calmette-Guérin vaccine nor infection by non-TB mycobacteria interferes with IGRA results.23
Despite the importance of the detection and treatment of LTBI to control TB in developing countries, few studies have evaluated the performance of IGRA testing in HIV-infected patients in countries with high TB incidence. Therefore, this study compared the ability of the IGRA with the TST to detect LTBI in a cohort of patients with HIV and determined the correlations between test results and outcomes.
Materials and methodsStudy populationThis was a prospective non-randomized cohort study conducted from August 2012 to August 2014 in a tertiary care academic center, Hospital de Clínicas/Universidade Federal do Paraná (HC/UFPR) in Curitiba, Southern Brazil. The Institutional Review Board approved this study (IRB: #02239612.4.0000.0096), and all included patients provided written informed consent.
Sample size calculation was based on an α error of 0.05 and β error of 0.10 (90% power). For these calculations, the discordance rate between the TST and IGRA was arbitrarily set at 30%. Considering that there were approximately 1400 HIV-positive patients treated at this hospital, a finite population correction factor was used to reduce the standard error. Incorporating these estimates into the equation yielded a sample size of 100. A total of 154 patients were enrolled.
Outpatients aged >18 years who attended the HIV reference clinics at HC/UFPR were invited to participate in this study. Patients currently undergoing chemoprophylaxis or treatment for TB and those with recent TST results were excluded. A standardized form was created for data collection, which included patient demographic, laboratory, and clinical information.
Participants were simultaneously submitted first to the venous blood collection for the IGRA and then to the TST. To exclude active TB, all HIV-positive patients with a positive TST result (>5mm) underwent chest radiography and sputum specimen analysis by acid-fast bacilli smear and culture. In excluded active disease cases, isoniazid was administered for 6 months.11,24 Chemoprophylaxis was not recommended for patients with negative TST results regardless of IGRA results according to the Brazilian guideline at the time of the study.4 CD4+ T lymphocyte counts were determined in all patients by flow cytometry with FACScalibur™ (Becton Dickinson Inc., San Jose, CA, USA) and the Point-Of-Care CD4 Alere Pima™ (Alere, Germany).
IGRAQuantiFERON® TB Gold In-Tube (Cellestis Limited, Victoria, Australia) was used according to the manufacturer's instructions. The results were interpreted using specific software provided by the manufacturer. A result was considered positive if there was IFN-γ production in the test tube indicated by an optical density ≥0.35IU/mL and ≥25% of that in the negative control tube. An indeterminate result was defined as low mitogen response (<0.5IU/mL), if the negative control tube showed an optical density >8IU/mL, or if the test tube optical density was <0.35IU/mL or <25% of that of the negative control tube.
TSTThe TST was carried out by intradermal administration of 0.1mL PPD-Rt 23 (Staten Serum Institute, Copenhagen, Denmark) to the anterior portion of the left forearm. The results were collected after 48–72h. The transverse diameter of TST induration was measured by a ruler, and readings ≥5mm were considered positive reactions. The same qualified professional performed all TSTs and readings.
Data analysisData were compiled using JMP version 5.2.1 (SAS Institute Inc., Cary, NC, USA) and analyzed using GraphPad Prism version 5.03 (GraphPad Software Inc., La Jolla, CA, USA). Baseline demographic and clinical characteristics with normal and non-normal distributions are presented as means±standard deviation and medians with interquartile ranges (IQRs), respectively. Fisher exact test, Student t-test, or the Mann–Whitney U-test was used where appropriate. All p-values are based on two-tailed comparisons, and the level of significance was set at p<0.05.
The operational characteristics and 95% confidence intervals (CIs) of the IGRA were calculated considering the TST as the gold standard. Cohen kappa coefficient was used to assess the degree of concordance between tests. The strength of agreement was considered poor, fair, moderate, substantial, and optimal for kappa values ≤0.20, 0.21–0.40, 0.41–0.60, 0.61–0.80, and 0.81–1.0, respectively.24 The correlation between tests was assessed by Spearman correlation.
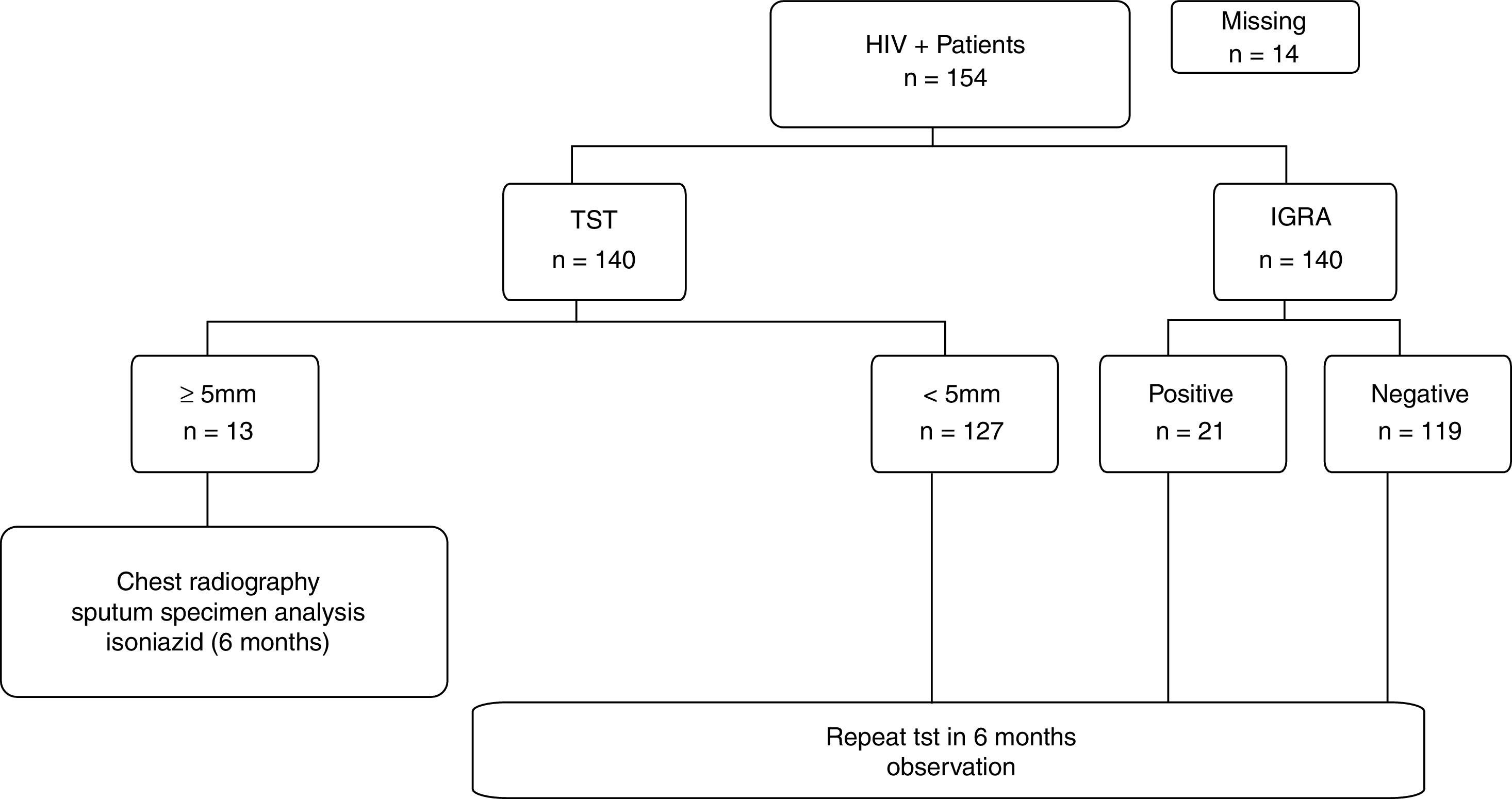
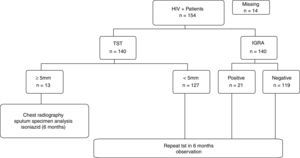
ResultsA total of 154 patients were enrolled. Among them, 14 (9%) did not return for the tuberculin test reading. One hundred fifteen (82%) patients had negative results on both tests. Discordant results were observed in 16 (11%) patients including 4 with positive TST and negative IGRA results, and 12 with negative TST and positive IGRA results. There was no statistical evidence that either method was different, because the rates of discrepancies were not significantly different (Fig. 1) (p=0.08).
Thirteen (9%) patients were diagnosed with LTBI on the basis of TST results, and isoniazid was administered for 6 months, with 100% adherence to the treatment. Considering the TST and IGRA results, a total of 25 (17%) patients presented with LTBI; thus, the IGRA detected 8% more cases (95% CI, 7.9–8.16%, p=0.0363). Patients with only positive IGRA results were not treated and are currently in follow-up.
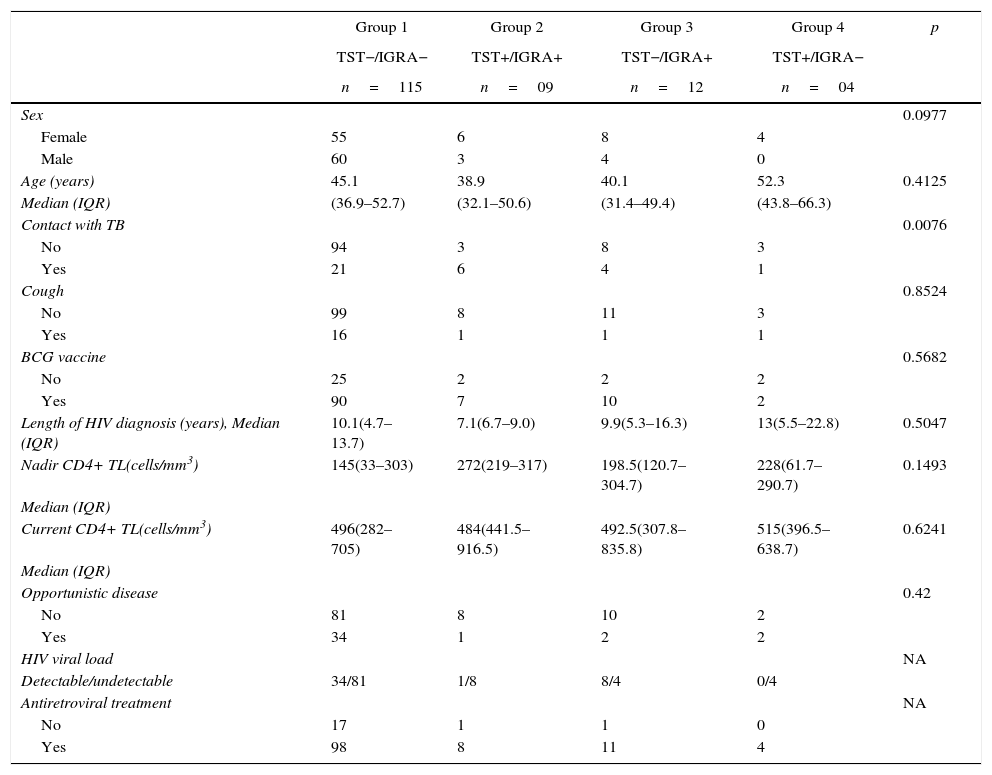
The patients were classified into 4 groups according to concordant and discordant results. Table 1 shows the epidemiological and clinical data from patients stratified according to the 4 combinations of TST and IGRA results.
Demographic, epidemiological, and clinical characteristics of study patients according to IGRA and TST results (N=140).
| Group 1 | Group 2 | Group 3 | Group 4 | p | |
|---|---|---|---|---|---|
| TST−/IGRA− | TST+/IGRA+ | TST−/IGRA+ | TST+/IGRA− | ||
| n=115 | n=09 | n=12 | n=04 | ||
| Sex | 0.0977 | ||||
| Female | 55 | 6 | 8 | 4 | |
| Male | 60 | 3 | 4 | 0 | |
| Age (years) | 45.1 | 38.9 | 40.1 | 52.3 | 0.4125 |
| Median (IQR) | (36.9–52.7) | (32.1–50.6) | (31.4–49.4) | (43.8–66.3) | |
| Contact with TB | 0.0076 | ||||
| No | 94 | 3 | 8 | 3 | |
| Yes | 21 | 6 | 4 | 1 | |
| Cough | 0.8524 | ||||
| No | 99 | 8 | 11 | 3 | |
| Yes | 16 | 1 | 1 | 1 | |
| BCG vaccine | 0.5682 | ||||
| No | 25 | 2 | 2 | 2 | |
| Yes | 90 | 7 | 10 | 2 | |
| Length of HIV diagnosis (years), Median (IQR) | 10.1(4.7–13.7) | 7.1(6.7–9.0) | 9.9(5.3–16.3) | 13(5.5–22.8) | 0.5047 |
| Nadir CD4+ TL(cells/mm3) | 145(33–303) | 272(219–317) | 198.5(120.7–304.7) | 228(61.7–290.7) | 0.1493 |
| Median (IQR) | |||||
| Current CD4+ TL(cells/mm3) | 496(282–705) | 484(441.5–916.5) | 492.5(307.8–835.8) | 515(396.5–638.7) | 0.6241 |
| Median (IQR) | |||||
| Opportunistic disease | 0.42 | ||||
| No | 81 | 8 | 10 | 2 | |
| Yes | 34 | 1 | 2 | 2 | |
| HIV viral load | NA | ||||
| Detectable/undetectable | 34/81 | 1/8 | 8/4 | 0/4 | |
| Antiretroviral treatment | NA | ||||
| No | 17 | 1 | 1 | 0 | |
| Yes | 98 | 8 | 11 | 4 |
IGRA, interferon-gamma release assay; TST, tuberculin skin test; BCG, Bacillus Calmette-Guérin; TB, tuberculosis; LTBI, latent tuberculosis infection; CDC, Centers for Disease Control and Prevention; CMV, cytomegalovirus; IQR, interquartile range; TL, T lymphocyte.
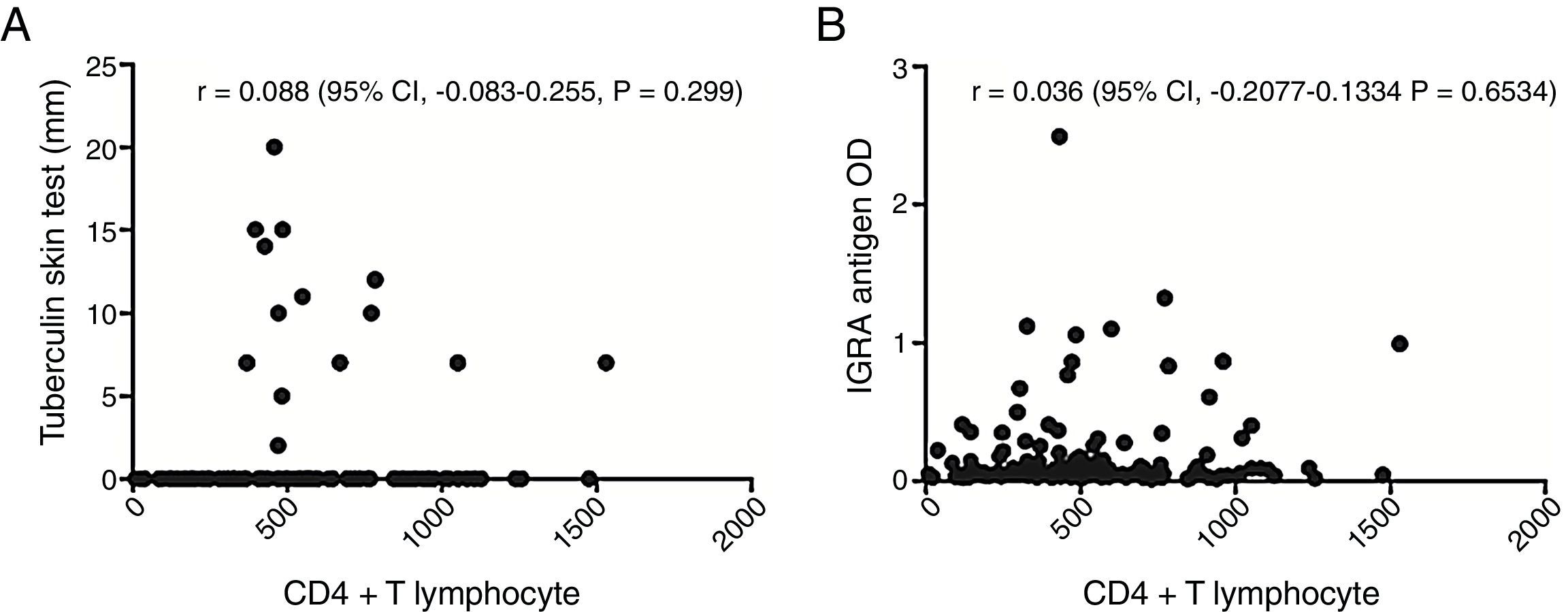
The assay results were related to CD4+ T cell count, and TST-positive results were detected only in patients with a CD4+ T lymphocyte count >300cells/mm3, while IGRA-positive results were observed in patients with low CD4+ T lymphocyte counts. However, only 21.5% of the studied patients had CD4+ T lymphocyte counts <300cells/mm3.
There was no influence of the presence of BCG vaccination in any of the two tests, but it should be noted that most patients were vaccinated in infancy (109/140–77.8%), i.e., immune stimulation occurred many years, which may have contributed to this finding.
Among the patients included in this study there was frequent reports of contact with people with TB, though most were asymptomatic, this should be seen as a warning sign to the physician who is performing the monitoring of this patient, as exposure to TB is a criteria to recommended LTBI treatment, regardless of TST results.
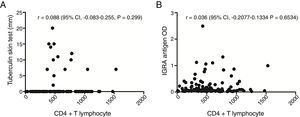
To investigate the relationship between the immune response against antigenic stimulation, the correlation between TST results (in mm), and the current CD4+ T lymphocyte counts was analyzed (Fig. 2A). In addition, the correlation between optical absorbance values obtained in the IGRA and current CD4+ T lymphocyte counts was evaluated (Fig. 2B). However, no correlations were detected.
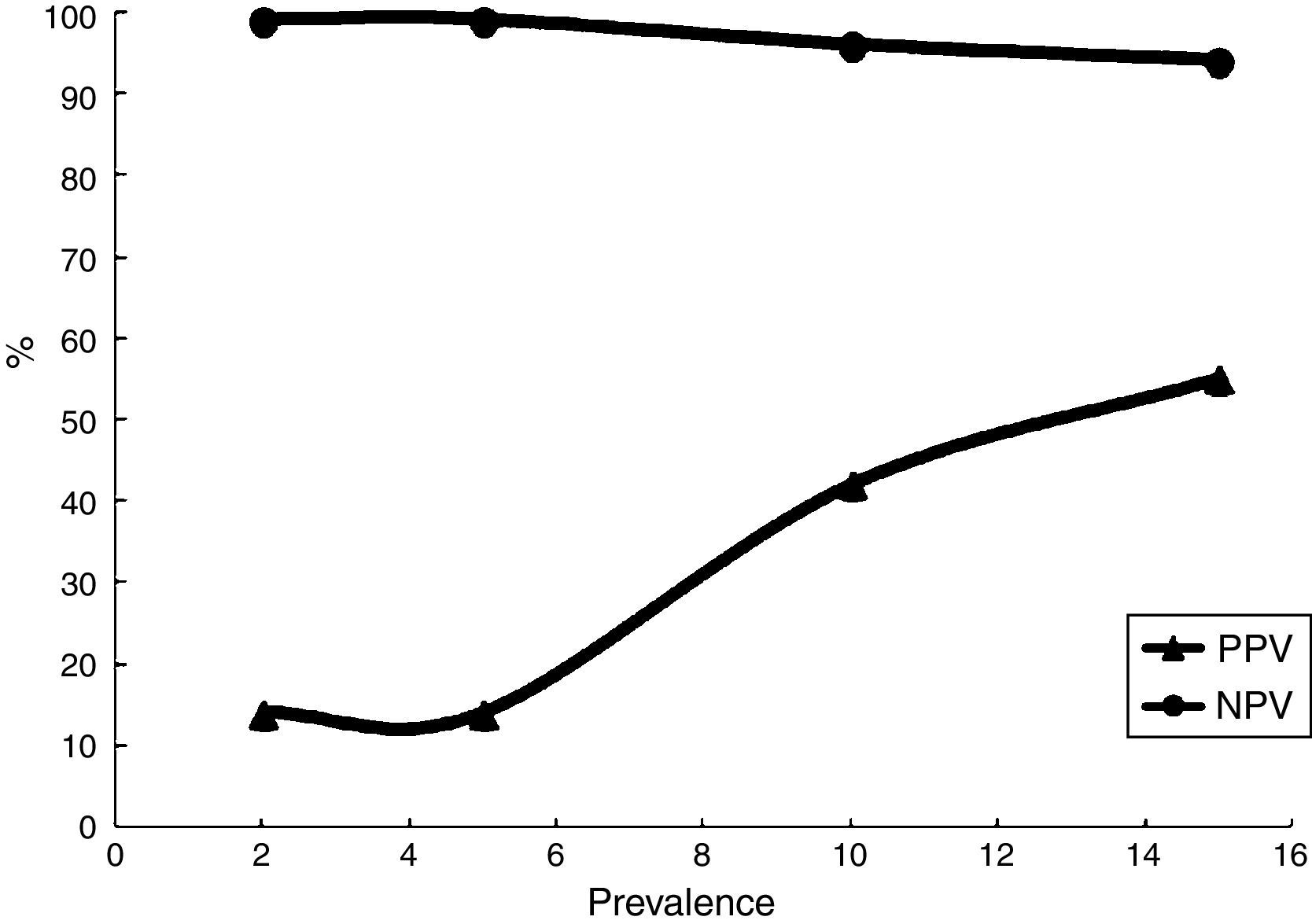
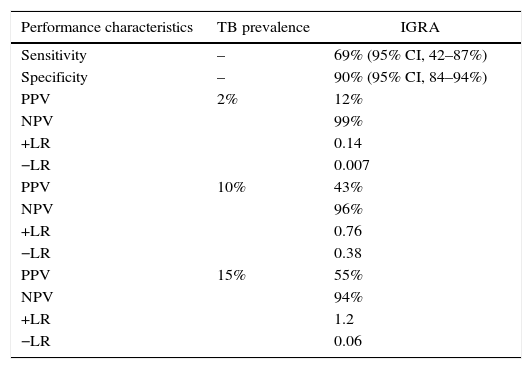
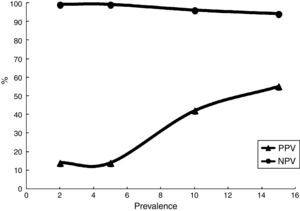
The agreement between the TST and IGRA was assessed using the Cohen kappa coefficient, which was found to be 0.214, indicating poor concordance. Considering TST results as the gold standard for LTBI diagnosis, the IGRA test performance was calculated: the sensitivity and specificity were 69% and 90%, respectively. To calculate the positive and negative predictive values, different prevalence of TB (2–15%) were evaluated; as expected, the positive predictive value varied considerably (Table 2) (Fig. 3).
IGRA performance characteristics with respect to TB prevalence.
| Performance characteristics | TB prevalence | IGRA |
|---|---|---|
| Sensitivity | – | 69% (95% CI, 42–87%) |
| Specificity | – | 90% (95% CI, 84–94%) |
| PPV | 2% | 12% |
| NPV | 99% | |
| +LR | 0.14 | |
| −LR | 0.007 | |
| PPV | 10% | 43% |
| NPV | 96% | |
| +LR | 0.76 | |
| −LR | 0.38 | |
| PPV | 15% | 55% |
| NPV | 94% | |
| +LR | 1.2 | |
| −LR | 0.06 |
PPV, positive predictive value; NPV, negative predictive value; +LR, positive likelihood ratio; −LR, negative likelihood ratio.
All patients were followed up for 2 years. One patient with negative results in both tests died from sepsis. Another patient with discordant results (TST−/IGRA+) presented with TST seroconversion and was started on chemoprophylaxis. There were no cases of active TB.
DiscussionMost studies that evaluated the performance of the IGRA for LTBI diagnosis were conducted in regions with low disease prevalence and primarily included HIV-negative individuals. These studies showed a good correlation between a positive test result and the presence of LTBI or even active TB.25–27 In the present study, the performance of the IGRA methodology was evaluated in HIV-positive patients living in a region with high TB prevalence. No patients with low CD4+ T lymphocyte counts had positive TST results, highlighting the difficulties of LTBI diagnosis in immunosuppressed patients. On the other hand, IGRA results were positive even in patients with lower CD4+ T lymphocyte counts despite the smaller number of patients. It is important to note that the majority of studied patients (78.5%) had CD4+ T lymphocyte counts >300cells/mm3. However, the proportion of IGRA positivity was similar regardless of CD4+ T lymphocyte count, similar findings were reported by Souza et al. (2014).28
Previous report comparing TST and IGRA results showed poor agreement, indicating that neither test can be used in place of the other.28 Thus, these methodologies should be used to complement each other; the method should be selected depending on patient characteristics. In HIV-positive patients, the TST should be performed only in individuals with CD4+ T lymphocyte counts >400cells/mm3, while the IGRA should be performed for those who have difficulty returning for a second visit, those recently vaccinated with Bacillus Calmette-Guérin, or those who recently underwent the TST.24
In this study, considering the results obtained by the TST and IGRA, the use of both techniques would increase the detection of LTBI patients from 9% (13 patients) to 17% (25 patients). IGRA and TST together detected an additional 8% of LTBI cases compared to TST alone. If these findings were confirmed, promptly administered LTBI treatment would reduce the number of patients with HIV/TB co-infection. However, further studies should be conducted to confirm the present conclusions as well as thoroughly evaluate the financial implications of this double testing protocol given the high cost of the IGRA.
The Centers for Disease Control guidelines does not recommend routine testing with both the TST and IGRA. However, the results of both tests can be useful when there are risks of infection or disease progression, especially in HIV-infected people or children aged <5 years who are exposed to a patient with infectious TB.27 On the basis of these findings, we emphasize the importance of the introduction of IGRA in routine laboratory tests for HIV-positive individuals with negative TST results, recommendation which was subsequently published by technical statement from the Brazilian Ministry of Health.29
In contrast to some previous reports,9,30–32 in the present study, there were no indeterminate results. Among the factors that may have contributed to this, some authors consider the importance of technical training, operational care, and attention to detail during the course of collection, transport, and handling of clinical specimens, because they can directly influence the results.33
Some reports show the IGRA could have better results than the TST in the identification of LTBI in patients with higher degrees of immunosuppression.23 In this study, the proportion of IGRA-positive results in severely immunocompromised patients was similar to that regarding TST results. However, the quantitative values (i.e., optical density for the IGRA and mm in the TST) obtained were not correlated with CD4+ T lymphocyte counts. It should be noted that the quantification of CD4+ T lymphocytes is just one way to assess cell immune response in HIV-positive patients. In the present study, no test was performed to qualitatively evaluate individual immune responses.
One of the main challenges when evaluating new LTBI diagnostic methods is the low sensitivity of the TST as the gold standard for comparison, which implies difficulties on PPV calculating. From regions with low TB prevalence and risk, sensitivity is usually calculated in patients with TB and specificity is measured using clinical data from patients who will be followed up and develop TB.34 Furthermore, the evaluation of these tests requires several individuals as well as prospective cohort studies comparing the presence of disease among individuals with positive and negative results over several years. Compared to the TST, such studies are costly, time consuming, and incur a significant risk of loss of patients during follow-up.
Therefore, this study evaluated the performance characteristics of the IGRA considering the TST as the gold standard. The sensitivity was 69%, which is similar to that in previous studies,35,36 and the specificity was good at 90%. The assessment of the predictive value of the IGRA is impaired, because LTBI is an asymptomatic infection. However, considering the distinct prevalence of TB, the IGRA has a low positive predictive value in settings with low TB prevalence, while in areas with high TB prevalence as secondary reference units, the test has better positive predictive values. During 2 years of follow-up, no cases of active TB were detected among the patients in the present study. Regardless, considering that a patient with LTBI (i.e., a positive TST result) presents an annual risk of developing active disease from 5% to 10%,6 a longer follow-up period is necessary to clarify if a positive IGRA result can predict progression to TB.
Systematic reviews and meta-analyses have been conducted to evaluate the performance characteristics of the IGRA. Rangaka et al. (2012) found that neither IGRA nor the TST has high accuracy for active TB prediction (incidence rate ratio: 2.11 for the IGRA and 1.6 for the TST at a cut-off of 10mm).35 Moreover, the IGRA positivity rate in countries with high disease burden and infection rates could indicate M. tuberculosis sensitization but might not be suitable for predicting TB. Diel et al. (2011) compared the accuracy of IGRAs with the TST for LTBI diagnosis and reported a pooled specificity and negative predictive value of 99.4% and 94%, respectively.36 Some studies evaluated the probability of developing active disease (i.e., positive predictive value for progression) in individuals with positive IGRA results, and the rate of progression observed ranged from 2% to 10%.36
In conclusion, the IGRA can be a complementary tool for LTBI diagnosis, especially in immunocompromised patients. The recognition and treatment of LTBI can reduce morbidity and mortality in HIV/TB co-infected patients; these tests should be performed early regardless of the degree of immunosuppression. However, the diagnosis of latent M. tuberculosis infection in people living with HIV/AIDS remains challenging. The low reactivity of the TST and impact of HIV/TB co-infection on HIV-positive patient survival highlight the need for the development and evaluation of new diagnostic methodologies.
Financial supportConselho Nacional de Pesquisa: Universal #478106/2011-4
Conflicts of interestThe authors declare no conflicts of interest.