The aim of this study was to identify highly oncogenic forms of human papillomavirus in the oral mucosa of asymptomatic men.
MethodsIn this study, we analyzed samples of exfoliated cells from the oral cavity of 559 asymptomatic men. DNA-human papillomavirus was detected using the consensus primers PGMY09/11; viral genotyping was performed using type-specific PCR and restriction fragment length polymorphism.
ResultsDNA-human papillomavirus was detected in 1.3% of the study participants and of those 42.8% were infected by more than one type of virus. Viral types included HPV6, 11, 89 (low oncogenic risk), and HPV52, 53 (high oncogenic risk). Increased vulnerability to human papillomavirus infection was observed in individuals aged over 26 years, among those who reported oral sex practices, and in those who have had more than 16 sexual partners since first engaging in sexual intercourse.
ConclusionsThere was a low prevalence of human papillomavirus detection in the oral mucosa of asymptomatic men. Highly oncogenic human papillomavirus types and infection by more than one viral type was observed. Oral sex practices and a large number of sexual partners may increase the risk of acquiring human papillomavirus infection.
The human papillomavirus (HPV) is a primary risk factor for cervical neoplasia and is associated with 25% of cancers affecting the head and neck region,1 and may induce development of oropharynx carcinoma.2
There are two forms of HPV, including high oncogenic risk (HR) and low oncogenic risk (LR) types. Low-risk types are associated with benign lesions in the host characterized as ordinary or condylomatous warts and include HPV6, 11, 40, 42, 43, 44, 54, 61, 70, 72, and 81. High-risk types have carcinogenic potential and include HPV 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 59, 66, 68, 72, and 81.3
The more an individual engages in risky behaviors such as oral sex and has high number of sexual partners, the greater the risk of acquiring the virus, with subsequent development of cellular differentiation and progression to neoplasia.4,5 However, some individuals develop oropharynx carcinoma without prior exposure to these risk factors, suggesting that potentially oncogenic viruses may lead to changes in control and cell proliferation mechanisms.6
Previous studies have examined the prevalence of HPV in the oral mucosa of asymptomatic men and suggested its association with neoplastic development; however, the assessment methods have shown disparate frequencies of infection, ranging from 0% to 100%.7 Thus, the aim of this study was to assess the frequency of highly oncogenic forms of HPV in the oral mucosa of asymptomatic men.
Methods and subjectsExfoliated cells from the oral cavity of 559 asymptomatic men were analyzed. Ethical approval was granted by the Ethics Committee in Research of UFMS, protocol CAAE 0251.0.049.000-11. All participants completed a questionnaire containing information regarding risk behaviors that may predispose them to HPV infection.
Collection of specimens and detection of HPV-DNASamples were collected through 5–10 brushings in regions of pre-established oral mucosa, including the right buccal mucosa (position from top to bottom); left buccal mucosa (position from top to bottom); right, left and dorsal side of the tongue; and inner regions of upper and lower lips. After collection, DNA was extracted from the samples using the Wizard Genomic DNA Purification kit (Promega, Fitchburg, WI, USA) and quantified using a NanoDrop (Thermo Scientific, Waltham, MA, USA) (180–260nm).
HPV-DNA detection was performed using PCR with a pool of consensus primers that amplify PGMY09/11 450-bp DNA sequences within the L1 region of HPV, as described previously.8 An endogenous control was used to verify DNA integrity using primers for the β-globin gene, PC04 and GH20, which amplify a 286-bp region of human DNA. Negative controls for background contamination were added to the DNA template. PCR products were analyzed using 1.5% agarose gel electrophoresis with ethidium bromide staining to visualize the DNA under ultraviolet (UV) light. Molecular weights were determined by comparison with a 100-bp DNA ladder.
Genotyping using type-specific PCR (TS-PCR) and restriction fragment length polymorphism (RFLP)HPV-DNA positive samples were genotyped by PCR using TS-PCR for the E6 and E7 gene DNA sequences of HPV 6, 11, 16, 18, 31, 33, and 45.9 PCR products were analyzed on a 2.5% agarose gel with ethidium bromide staining to visualize DNA under UV light. Molecular weights were determined by comparison with 100-bp and 50-bp DNA ladders. The same samples were analyzed using RFLP. Next, the PGMY 09/11 PCR product of these samples was purified from the agarose gel using the QIAEX II Gel Purification Kit Qiagen (Hilden, Germany) according to the manufacturer's protocol. The concentration of extracted materials was determined using a NanoDrop (180–260nm); samples containing the PCR product were subjected to enzymatic digestion for one hour at 37°C. The enzymes used for reaction included BamHI, DdeI, HaeIII, HinfI, RsaI, PstI, and Sau3A. The digestion pattern was analyzed on a 3% agarose gel with ethidium bromide under UV light and interpreted using an algorithm described previously.10
Statistical analysisThe distribution of positivity for HPV-DNA was investigated according to age range (≤25 years and ≥26 years), marital status, report of oral sex practices, and estimated number of sexual partners. Statistical analysis was performed using SPSS, version 10.011 of Pearson χ2 test for contingency tables, adjusted for Phi Cramer's V. To compare frequencies between age subgroups, when significant, the G test corrected by Yates index was used. The proportion of positive findings within the group was analyzed using the binomial test for two independent samples.
ResultsSamples of asymptomatic oral mucosa from men aged 18–68 years (mean, 23 years) was analyzed. Of the 559 samples collected, 514 (91.9%) were positive for the β-globin gene and thus included in the study. HPV-DNA was detected in seven samples, which accounted for 1.3% of the study participants.
Detected viral types included HPV6, 11, and 89 in the LR group and HPV52 and 53 in the HR group (Figs. 1 and 2). Highly oncogenic infection was detected in two out of the seven samples (28.5%), and infection by more than one viral type was found in three of the seven samples (42.8%).
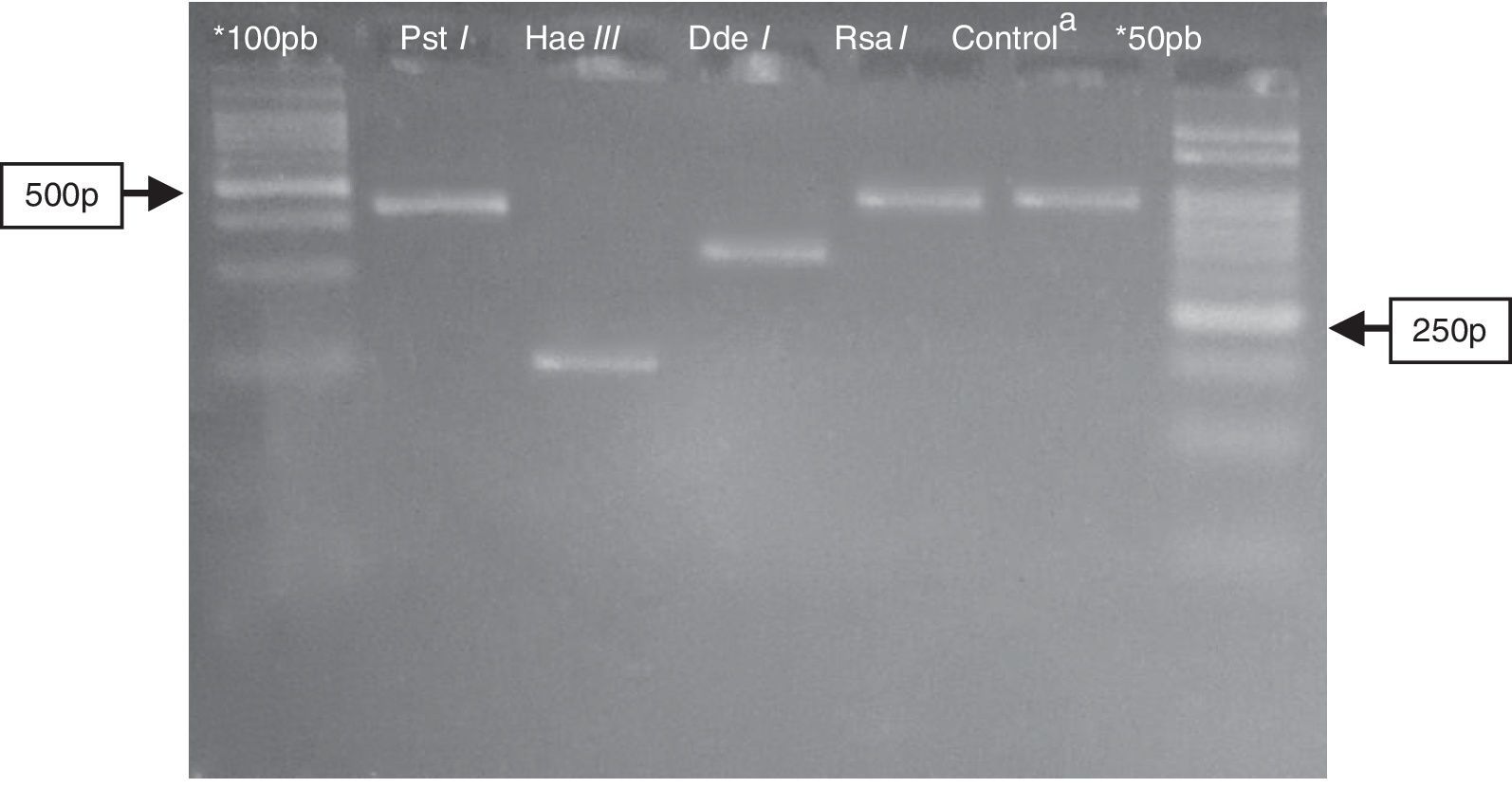
Electrophoresis in agarose 3% showing patterns of polymorphism of the restriction fragments obtained with the product of PGMY09/11 used for genotyping of the HPV52 (HR). *100pb and 50pb=ladder DNA; acontrol: PGMY09/11 PCR product of 450pb without enzymatic digestion. Restriction enzyme: Pst I=not digestion; Hae III≅200pb; Dde I≅350pb; Rsa I=not digestion.
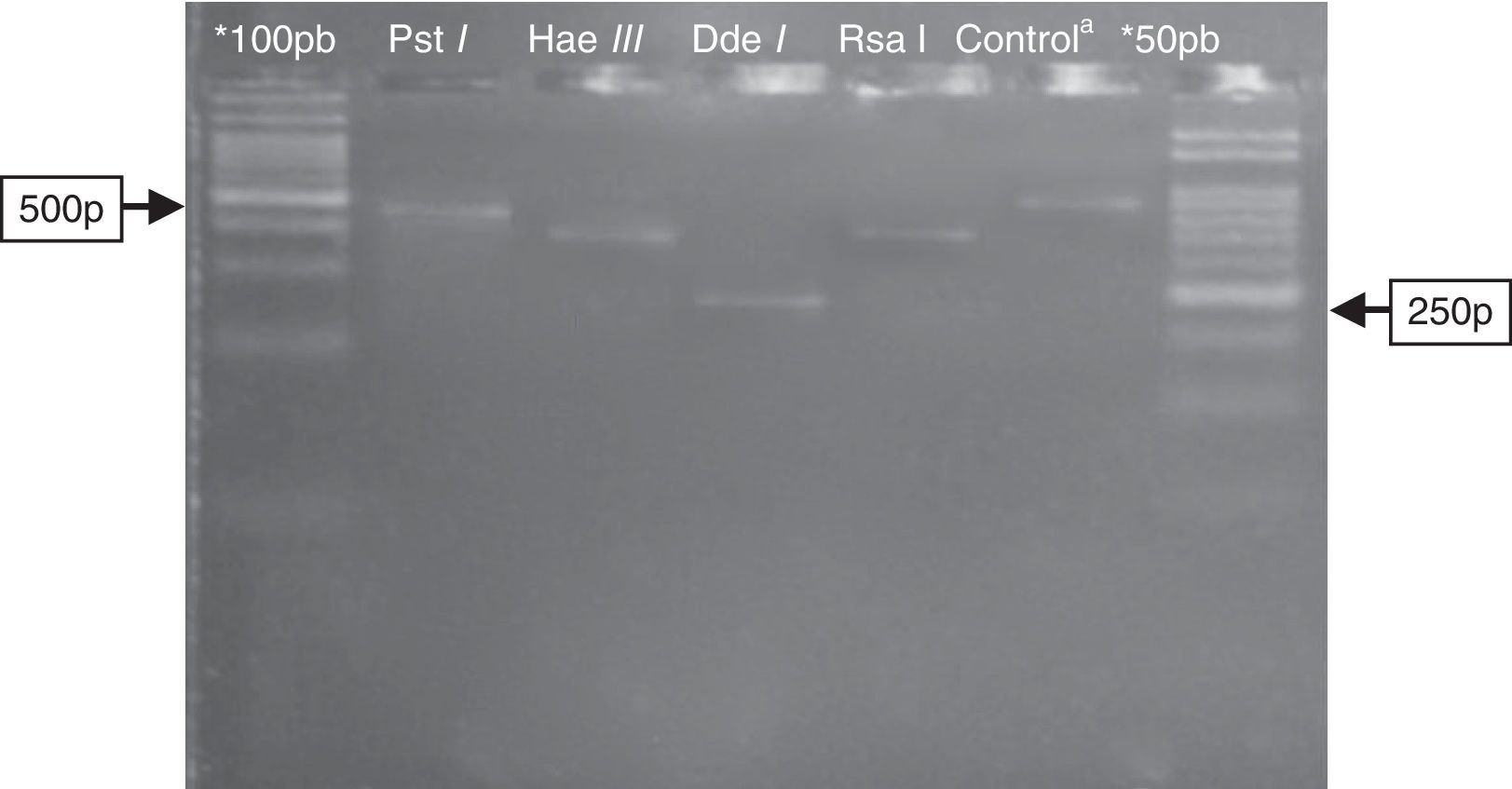
Electrophoresis in agarose 3% showing patterns of polymorphism of the restriction fragments obtained with the product of PGMY09/11 used for genotyping of the HPV89 (LR). *100pb and 50pb=ladder DNA; acontrol: PGMY09/11 PCR product of 450pb without enzymatic digestion. Restriction enzyme: Pst I=not digestion; Hae III≅350pb; Dde I≅250pb; Rsa I≅380pb.
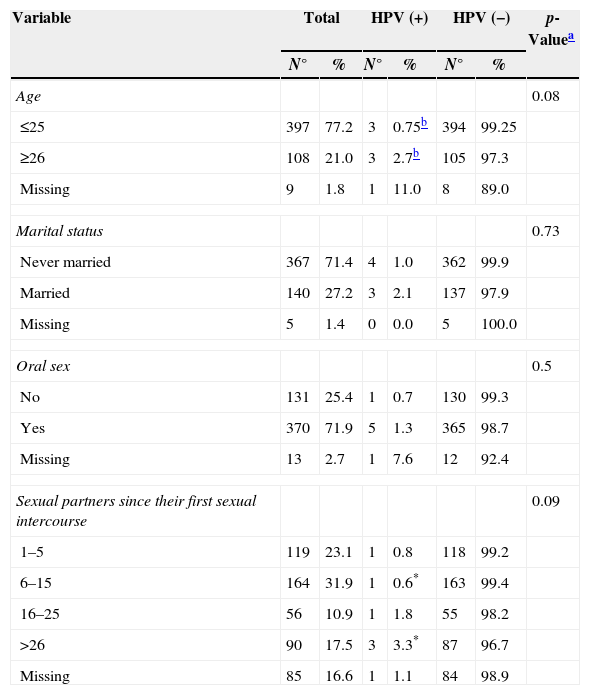
Among the participants, 71.7% reported being single and 27.2% married, with a higher HPV-DNA positivity among married men. The binomial test showed that individuals older than 26 years of age were more vulnerable to infection (p<0.01). Oral sex practices were reported by 71.9% of the respondents (Table 1), particularly among those over 26 years of age (p<0.01), where HPV-DNA positivity was higher.
Epidemiological characteristics of the population associated with positivity for HPV-DNA.
| Variable | Total | HPV (+) | HPV (−) | p-Valuea | |||
|---|---|---|---|---|---|---|---|
| N° | % | N° | % | N° | % | ||
| Age | 0.08 | ||||||
| ≤25 | 397 | 77.2 | 3 | 0.75b | 394 | 99.25 | |
| ≥26 | 108 | 21.0 | 3 | 2.7b | 105 | 97.3 | |
| Missing | 9 | 1.8 | 1 | 11.0 | 8 | 89.0 | |
| Marital status | 0.73 | ||||||
| Never married | 367 | 71.4 | 4 | 1.0 | 362 | 99.9 | |
| Married | 140 | 27.2 | 3 | 2.1 | 137 | 97.9 | |
| Missing | 5 | 1.4 | 0 | 0.0 | 5 | 100.0 | |
| Oral sex | 0.5 | ||||||
| No | 131 | 25.4 | 1 | 0.7 | 130 | 99.3 | |
| Yes | 370 | 71.9 | 5 | 1.3 | 365 | 98.7 | |
| Missing | 13 | 2.7 | 1 | 7.6 | 12 | 92.4 | |
| Sexual partners since their first sexual intercourse | 0.09 | ||||||
| 1–5 | 119 | 23.1 | 1 | 0.8 | 118 | 99.2 | |
| 6–15 | 164 | 31.9 | 1 | 0.6* | 163 | 99.4 | |
| 16–25 | 56 | 10.9 | 1 | 1.8 | 55 | 98.2 | |
| >26 | 90 | 17.5 | 3 | 3.3* | 87 | 96.7 | |
| Missing | 85 | 16.6 | 1 | 1.1 | 84 | 98.9 | |
HPV, human papillomavirus.
Among individuals who reported having had more than 16 sexual partners since their first sexual intercourse, HPV-DNA positivity was 2.8%.
DiscussionIn this study HPV was detected in 1.3% of the participants. A cohort study conducted in the US from 2000 to 2006 assessing the presence of HPV in oral mucosa found an HPV frequency of 6.0% in oral brushing and oropharyngeal scrapings.12 Other multicenter studies conducted to examine male subjects in Brazil, the US, and Mexico found a positivity of 2.1%, 3.6%, and 5.9%, respectively, in material from gargling.13
Previous studies have shown that the rate of HPV infection is high in male subjects,14 ranging from 3.5% to 45% for all detectable types.15 However, other studies show different values, with higher and more divergent prevalence.16 These results may reflect methodological discrepancies used for HPV-DNA detection, the reproducibility of genotyping assays, and the sensitivity and specificity of primers used for diagnosis.
The global distribution of different HPV types exhibits large variation, even for the oral cavity of subjects in different geographical regions. Studies examining HPV prevalence in the US conducted in 2009–2010 showed that among HR types in the oral mucosa, the HPV genotypes 16, 66, and 51 were prevalent, and among LR types, 62, 55, and 89 were predominant,5 in contrast to the findings of this study. Other authors demonstrated that HPV types 6, 11, and 16 were the most prevalent.16
The oral mucosa of healthy individuals can host approximately 4.4% of carcinogenic types and 7.5% of non-carcinogenic types of HPV.7 The difference between HPV types detected in the oral cavity and cervical–vaginal region shows a broader view of distribution for different tissue types. Thus, these regions can be colonized by types not previously detected, which may explain events such as the lack of cell cycle control in these tissues.
In men, the prevalence of infection is not related to age, nor is the prevalence higher in younger individuals, in contrast to trends observed in women. These findings suggest that men may experience persistent long-term infection with a high rate of re-infection. Other studies have revealed that HPV positivity increases with age.5,13,17
The present study observed higher positivity among individuals aged more than 26 years; however, this may be associated with the adoption of risky sexual behavior by this age group.
Oral sex practices have been widely associated with the risk of HPV infection, although it is impossible to identify an independent factor responsible for infection.1,18 Our study showed that oral sex practices were prevalent in 71.9% of participants. A study conducted in Mexico examined the incidence of HPV in the oral mucosa of women with cervical lesions who reported oral sex practices and also showed 72% positivity for HPV.19
Despite the hypothesis that HPV is transmitted to a particular region through oral sex practices, there is no consensus for collecting participants’ secondary data. Thus, studies minimizing sample failures, such as the effect of mouth kissing, which is associated with infection among those who reported no oral sex practices, should be examined.12
The number of sexual partners may be a marker of other risk factors.20 Previous studies have analyzed the correlation between HPV infection in couples and in men with a large number of sexual partners.18,21 The present study showed a higher positivity of HPV among individuals with a larger number of sexual partners; however, this observation should not be considered in isolation since a combination of practices predisposing to infection must be considered.
In conclusion, the three primary findings of this study include a low prevalence of HPV infection in the oral mucosa of asymptomatic men, the ability of the mouth mucosa to host high-risk HPV types, and the presence of multiple viral types without always presenting with clinical lesions.
Risky sexual behaviors such as oral sex and having a large number of sexual partners may be related to individual predisposition to HPV infection, making them more vulnerable to HPV infection.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by Foundation to Support Development of Education, Science and Technology of the State of Mato Grosso do Sul, Brazil (Fundect-MS), National Research Council (CNPq), and Coordination of Improvement of Higher Level (CAPES).