Survival of patients with acquired immune deficiency syndrome has improved with combination antiretroviral therapy; mortality due to liver diseases, however, has also increased in these patients.
ObjectivesTo estimate the accumulated probability of survival in human immunodeficiency virus–hepatitis C virus coinfected and non-coinfected patients and to investigate factors related to acquired immune deficiency syndrome patients’ survival.
MethodsNon-concurrent cohort study using data from surveillance information systems of acquired immune deficiency syndrome patients over 13 years of age. Hepatitis C and B, human immunodeficiency virus exposure category, CD4+ T cell count, age group, schooling, race, sex, and four acquired immune deficiency syndrome diagnosis periods were studied. Kaplan–Meier survival analysis and Cox model with estimates of the hazard ratio and 95% confidence interval were used.
ResultsOf the total 2864 individuals included, with median age was 35 years, 219 died (7.5%), and 358 (12.5%) were human immunodeficiency virus–hepatitis C virus coinfected. The accumulated probability of survival in human immunodeficiency virus–hepatitis C virus coinfected patients, after acquired immune deficiency syndrome diagnosis, at 120 months, was 0%, 38.9%, 83.8% in 1986–1993, 1994–1996, 1997–2002, respectively, and 92.8% at 96 months in 2003–2010; survival in non-coinfected patients at 120 months was 80%, 90.2%, 94% in 1986–1993, 1994–1996, 1997–2002, respectively, and 94.1% at 96 months in 2003–2010. In the multivariate model the following variables were predictive of death: hepatitis C virus coinfection (hazard ratio=2.7; confidence interval 2.0–3.6); Hepatitis B virus coinfection (hazard ratio=2.4; confidence interval 1.7–3.6); being ≥50 years old (hazard ratio=2.3; confidence interval 1.3–3.8); having 8–11 years of schooling (hazard ratio=1.6; confidence interval 1.1–2.3), having 4–7 years of schooling (hazard ratio=1.9; confidence interval 1.3–2.8) and having up to 3 years of schooling (hazard ratio=3.3; confidence interval 2.0–5.5).
ConclusionsAmong patients diagnosed after 1996, there was a significant increase in the cumulative probability of survival in human immunodeficiency virus–hepatitis C virus coinfected individuals; among those diagnosed with acquired immune deficiency syndrome from 2003 to 2010, this probability was similar between coinfected and non-coinfected patients.
The United Nations Program on human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS) states that “globally, 34.0 million (31.4 million–35.9 million) people were living with HIV at the end of 2011”, or 0.8% of adults aged 15–49 years.1 Survival of patients living with the AIDS has increased in the highly active antiretroviral therapy (HAART) era, currently called combination antiretroviral therapy (cART).2
In Brazil, the median survival time estimated was 5.1 months between 1982 and 19893 and increased to 58 months in 1996;4 in the south and southeast regions of Brazil, 59.4% of AIDS patients survived 108 months in 1998–1999,5 and in São Paulo, the accumulated probability of survival was 72% at 108 months in 1997–2003.6
Although cART has brought longer survival to HIV-infected patients, the morbidity and mortality due to viral hepatitis, especially type C, has also increased.7,8 It is estimated that about one-third of HIV-infected individuals in the world have hepatitis C virus (HCV) coinfection.9 In fact, decompensated liver disease due to HCV has increased as cause of death in patients with HIV–HCV coinfection,7 and the prevalence of cirrhosis10 and of deaths due to hepatocellular carcinoma have also been increasing in HIV-infected patients.10
The objective of the present study was to estimate the cumulative probability of survival after AIDS diagnosis in HIV–HCV-coinfected and non-coinfected patients and to perform exploratory analysis to investigate factors related to AIDS patients’ survival.
MethodsThis is a longitudinal observational study, based on medical records, of a non-concurrent cohort, of patients receiving care in a public referral center for the treatment of sexually transmitted diseases and AIDS in São Paulo city, Brazil (CRT DST/AIDS-SP, CRT). This clinic has also become a referral center for hepatitis treatment since 2004, and it is also the head office of the São Paulo State Program for Sexually Transmitted Diseases and AIDS.
The Metropolitan Region of São Paulo city had, in 2010, a population of 19.667.558 inhabitants according to local census,11 and 74,308 AIDS cases notified from 1980 to 2009.12 The sample included in this study comprises all AIDS cases in individuals aged 13 years or older being followed in our referral center, and with complete medical records.
Patients were excluded from the present study if he/she had a diagnosis of AIDS related complex (syndrome including fatigue and swollen lymph nodes) or if the death certificate stated AIDS as the cause of death without a laboratory exam to confirm HIV infection. These cases were more common before 1996, especially before 1987 (HIV viruses were identified in 1983 and 1986).13–15 Pregnancy was also a criteria of exclusion in this study.
For the purpose of inclusion in this study, HCV infection was defined by serological tests (ELISA or EIA, in any generation) and the qualitative or quantitative detection of HCV RNA, by the time of AIDS diagnosis or in the nearest date (up to two years before or after).
The sources of data in this study were the national notification databases for AIDS cases (SINAN Windows up to 2006 and SINAN Net from 2007 on), the CRT-Epidemiological Surveillance System, and the CRT-Laboratory System. A fourth source was the São Paulo State surveillance system, called BIP-AIDS (integrating SIM, Sistema de Informação sobre Mortalidade, civil notary offices and SEADE Foundation, Fundação Sistema Estadual de Análise de Dados). When some information was not available in these electronic systems, it was searched directly from medical records.
A special spreadsheet was created for data collection including the following independent variables: AIDS diagnosis periods (1986–1993, 1994–1996, 1997–2002 and 2003–2010), patient's age, gender, ethnicity/race, years of schooling, CD4+ T cell count at AIDS diagnosis, HCV infection and hepatitis B surface antigen (HBsAg) in a period next to AIDS diagnosis (up to two years before or after). The elapsed time from diagnosis until death, in months, was taken as the dependent variable.
The source of exposure to HIV was registered in three categories: from heterosexual relationship, men who have sex with men (MSM), and by the use of intravenous drugs (IDU). Transmission by blood transfusions, accidents and others were excluded from the survival analysis. When information was initially registered as “unknown”, the original medical record in the clinic was searched manually for completion.
The final date for survival calculation was established as April 30, 2011. Loss to follow up and death by other-than-AIDS or unknown causes were censored (incomplete follow up).
Statistical analysisInitially, a descriptive analysis was performed, presenting absolute and relative frequencies, comparing patients infected and uninfected with HCV, observing distributions and characteristics of users in relation to the study variables of interest. Chi-square test was used to compare infected and uninfected cases by HCV.
Kaplan–Meier analysis of survival was performed, with a cumulative probability of survival with AIDS estimated in months, according to each variable of interest and period of diagnosis of AIDS. Statistical significance was assessed by the log rank test. A Cox regression or proportional hazards model was chosen to calculate risk or hazard ratio (HR) in survival analysis, with a confidence interval (CI) of 95%, and the variable “period of diagnosis” was used as a stratum. Univariate analysis was followed by multivariate analysis. Associations were considered statistically significant with a significance level of less than 5%. Microsoft Excel 2003 and STATA software, version 10.0, were used for the statistical analysis.
The study protocol was designed in accordance with the National Health Committee guidelines, and was approved by CRT's and Faculdade de Saúde Pública – São Paulo University's Ethics Committees with the protocol number CRT 002/2010 and FSP-USP 44/2010.
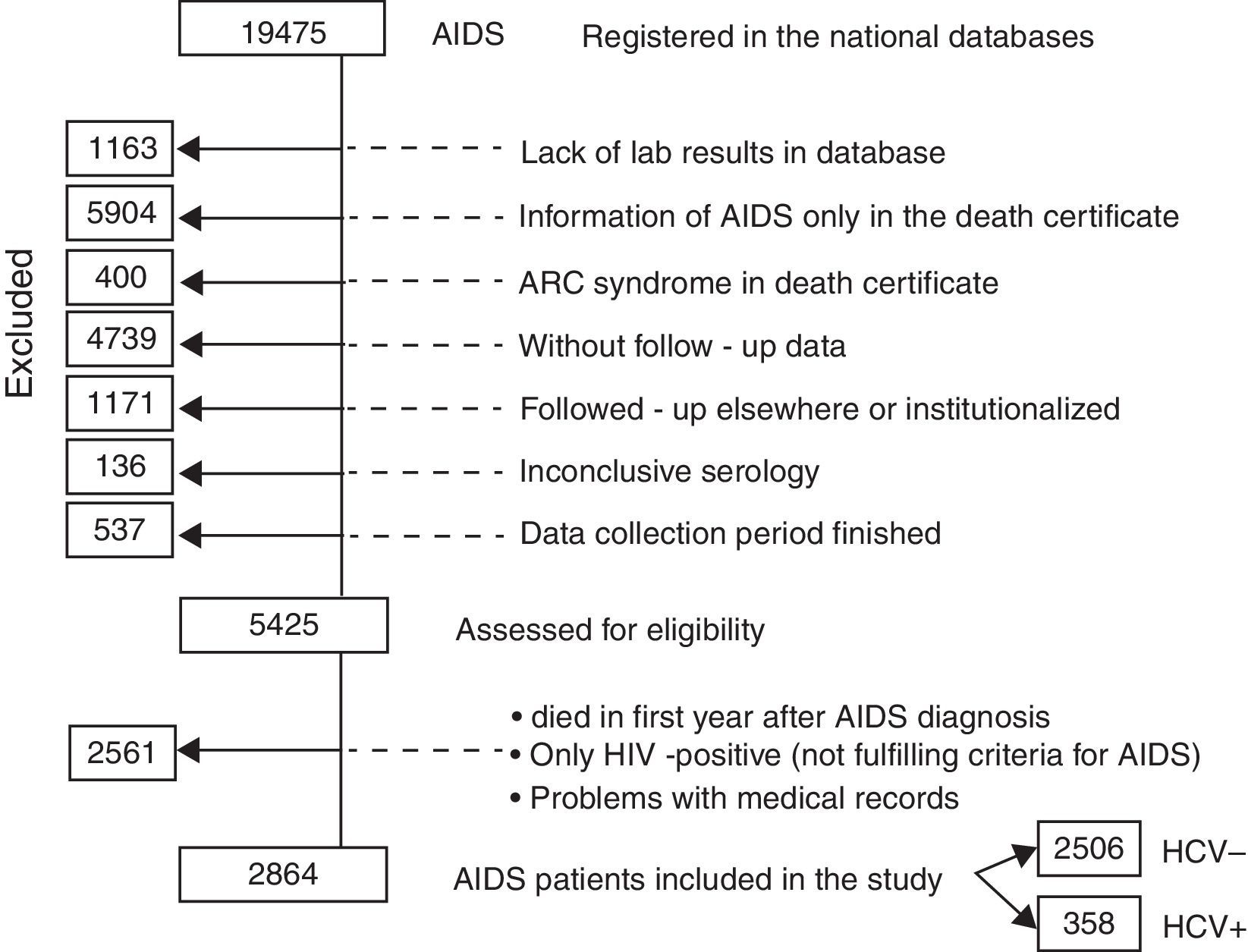
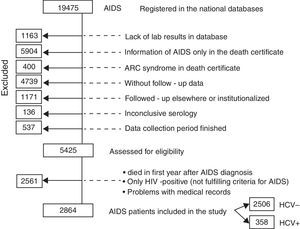
ResultsFrom July 1986 to April 2010, a total of 19,475 AIDS cases were registered in the major national databases (SINAN Windows and Net). However, 14,050 needed to be excluded from the study for the reasons shown in Fig. 1 and described in detail below. The remaining 5425 AIDS cases were therefore assessed for eligibility: 2561 were further excluded because they died in first year of follow-up after AIDS diagnosis, because they did not fulfill the criteria for AIDS characterization, or because of problems with the medical records (such as the lack of an identifying number). Another reason for exclusion was the lack of a HCV RNA test for hepatitis C confirmation (hepatitis was registered as cirrhosis). Therefore, this study is based on 2864 cases of AIDS patients, among whom 358 were coinfected with HCV.
The reasons for exclusions were: lack of laboratory exams in the database; medical records bringing information of AIDS only in the death certificate, or, otherwise, alleged ARC symptoms in the death certificate, without clinical or lab exams; cases not registered in the CRT Epidemiological surveillance system database, which contains follow-up data; patients being followed-up in other institutions, admitted in hospitals, or subjects of clinical research protocols; inconclusive serology and finally, patients were excluded when the period for data collection was finished.
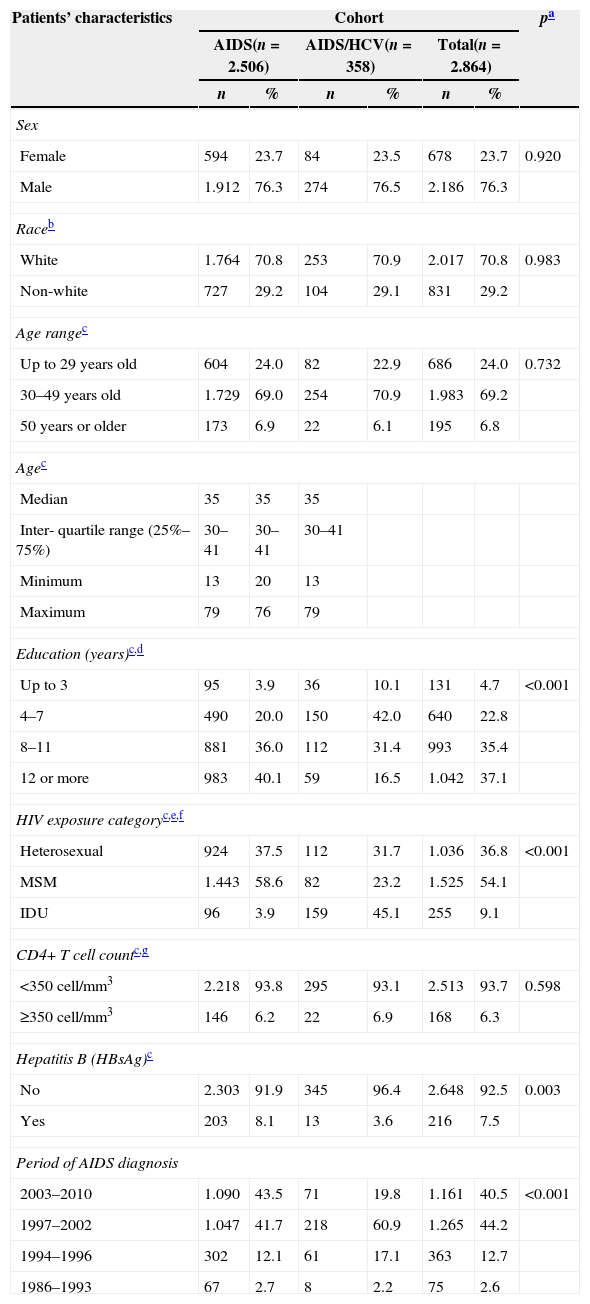
Table 1 summarizes the patients’ demographic and clinical characteristics among the 2864 AIDS cases, infected or not with HCV.
Characteristics of patients according to HCV infection (CRT-DST/AIDS-SP, 1986–2010).
| Patients’ characteristics | Cohort | pa | |||||
|---|---|---|---|---|---|---|---|
| AIDS(n=2.506) | AIDS/HCV(n=358) | Total(n=2.864) | |||||
| n | % | n | % | n | % | ||
| Sex | |||||||
| Female | 594 | 23.7 | 84 | 23.5 | 678 | 23.7 | 0.920 |
| Male | 1.912 | 76.3 | 274 | 76.5 | 2.186 | 76.3 | |
| Raceb | |||||||
| White | 1.764 | 70.8 | 253 | 70.9 | 2.017 | 70.8 | 0.983 |
| Non-white | 727 | 29.2 | 104 | 29.1 | 831 | 29.2 | |
| Age rangec | |||||||
| Up to 29 years old | 604 | 24.0 | 82 | 22.9 | 686 | 24.0 | 0.732 |
| 30–49 years old | 1.729 | 69.0 | 254 | 70.9 | 1.983 | 69.2 | |
| 50 years or older | 173 | 6.9 | 22 | 6.1 | 195 | 6.8 | |
| Agec | |||||||
| Median | 35 | 35 | 35 | ||||
| Inter-quartile range (25%–75%) | 30–41 | 30–41 | 30–41 | ||||
| Minimum | 13 | 20 | 13 | ||||
| Maximum | 79 | 76 | 79 | ||||
| Education (years)c,d | |||||||
| Up to 3 | 95 | 3.9 | 36 | 10.1 | 131 | 4.7 | <0.001 |
| 4–7 | 490 | 20.0 | 150 | 42.0 | 640 | 22.8 | |
| 8–11 | 881 | 36.0 | 112 | 31.4 | 993 | 35.4 | |
| 12 or more | 983 | 40.1 | 59 | 16.5 | 1.042 | 37.1 | |
| HIV exposure categoryc,e,f | |||||||
| Heterosexual | 924 | 37.5 | 112 | 31.7 | 1.036 | 36.8 | <0.001 |
| MSM | 1.443 | 58.6 | 82 | 23.2 | 1.525 | 54.1 | |
| IDU | 96 | 3.9 | 159 | 45.1 | 255 | 9.1 | |
| CD4+ T cell countc,g | |||||||
| <350cell/mm3 | 2.218 | 93.8 | 295 | 93.1 | 2.513 | 93.7 | 0.598 |
| ≥350cell/mm3 | 146 | 6.2 | 22 | 6.9 | 168 | 6.3 | |
| Hepatitis B (HBsAg)c | |||||||
| No | 2.303 | 91.9 | 345 | 96.4 | 2.648 | 92.5 | 0.003 |
| Yes | 203 | 8.1 | 13 | 3.6 | 216 | 7.5 | |
| Period of AIDS diagnosis | |||||||
| 2003–2010 | 1.090 | 43.5 | 71 | 19.8 | 1.161 | 40.5 | <0.001 |
| 1997–2002 | 1.047 | 41.7 | 218 | 60.9 | 1.265 | 44.2 | |
| 1994–1996 | 302 | 12.1 | 61 | 17.1 | 363 | 12.7 | |
| 1986–1993 | 67 | 2.7 | 8 | 2.2 | 75 | 2.6 | |
MSM, men who have sex with men; IDU, injecting drug users; HBsAg, surface antigen of hepatitis B.
In our study, 76.3% of the patients were men, 70.8% of white race, 69.2% aged 30–49 years with a median age of 35 years (minimum 13 and maximum 79), 58.2% with 4–11 years of schooling, 54.1% were MSM, 93.7% had CD4+ T cell count <350cell/mm3, 12.5% were HIV–HCV coinfected, 7.5% had Hepatitis B, and 44.2% of patients were AIDS diagnosed in the 1997–2002 period.
Regarding schooling years, 42% of coinfected individuals had 4–7 years of schooling while 40.1% of non-coinfected patients had 12 school years or more. The IDU HIV exposure category was identified in 45.1% among coinfected subjects while 58.6% of non-coinfected was MSM. Hepatitis B was more common in non-coinfected HIV–HCV (8.1% versus 3.6%; p=0.003).
Among the 2864 AIDS patients, 219 (7.6%) died between 1986 and 2010.
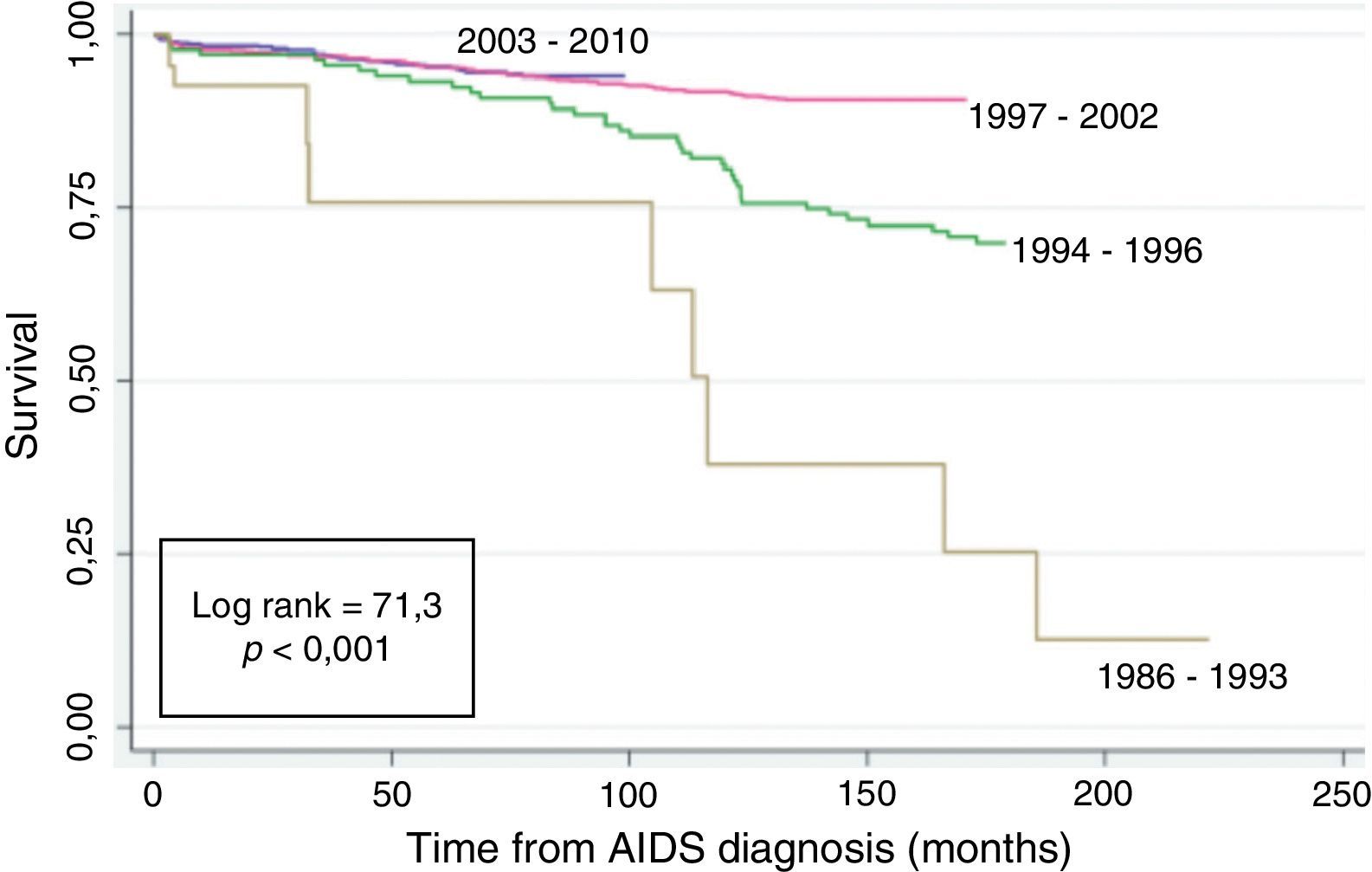
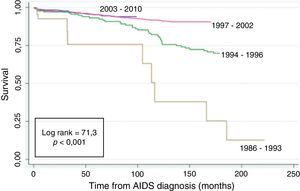
Fig. 2 shows the Kaplan–Meier survival curves of the patients according to the AIDS diagnosis period. A higher rate of survival was seen in the post-cART: 1997–2002 and 2003–2010 AIDS diagnosis period (log rank=71.3; p<0.001).
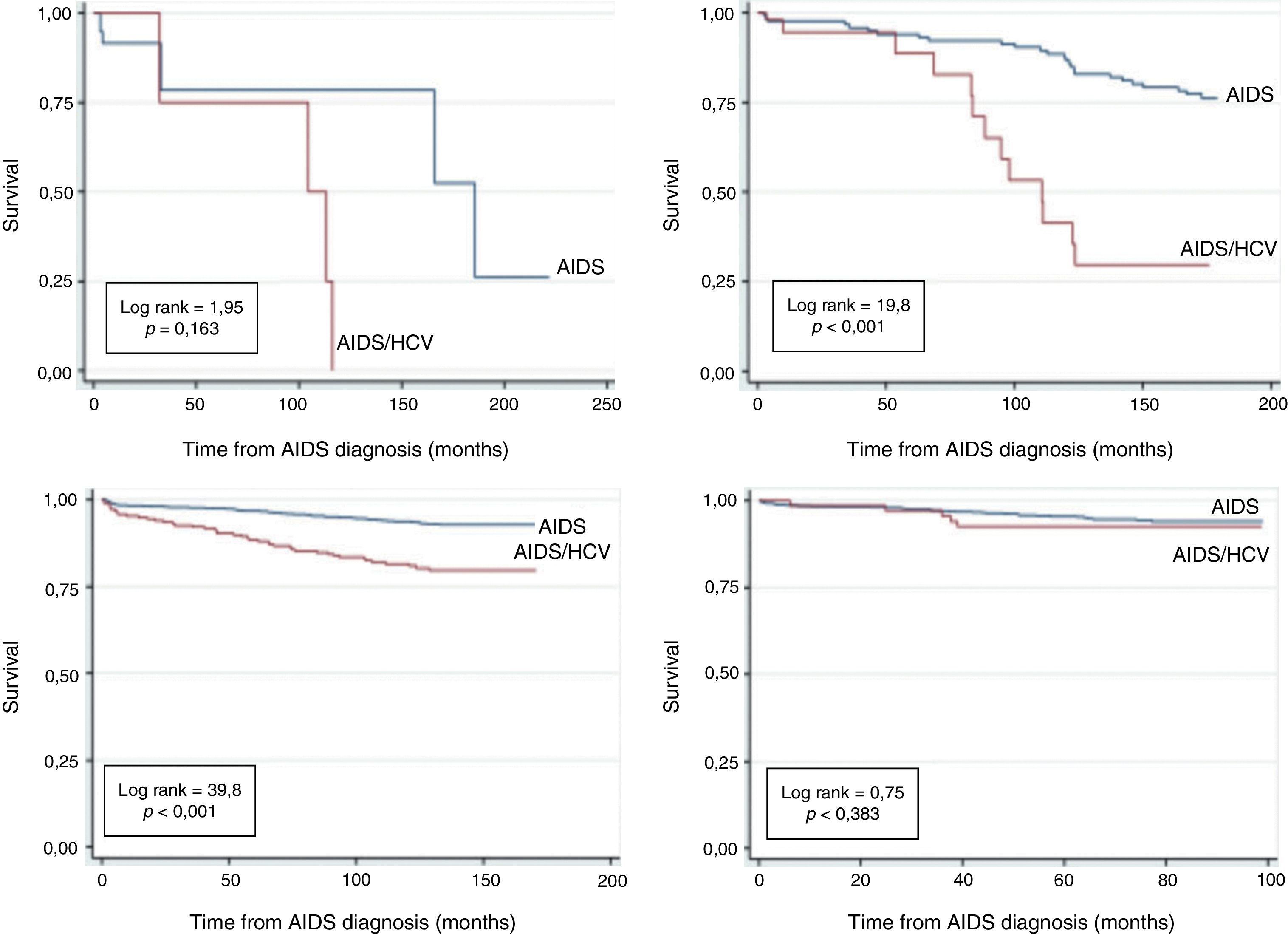
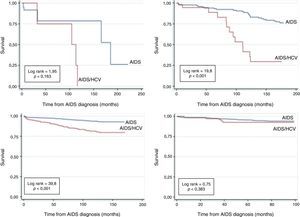
Fig. 3 shows the survival analysis of monoinfected and HIV–HCV coinfected AIDS patients according to AIDS diagnosis period. The accumulated probability of survival among coinfected patients at 120 months after AIDS diagnosis was 0% for those diagnosed in the period between 1986 and 1993, 38.9% in 1994–1996 and 83.8% in the 1997–2002 period. Survival in non-coinfected patients at 120 months was 80%, 90.2%, 94% in 1986–1993, 1994–1996, 1997–2002, respectively. Finally, in the 2003–2010 AIDS diagnosis period, because of a shorter observation time, the survival among coinfected was 92.8% and among non-coinfected was 94.1% at 96 months.
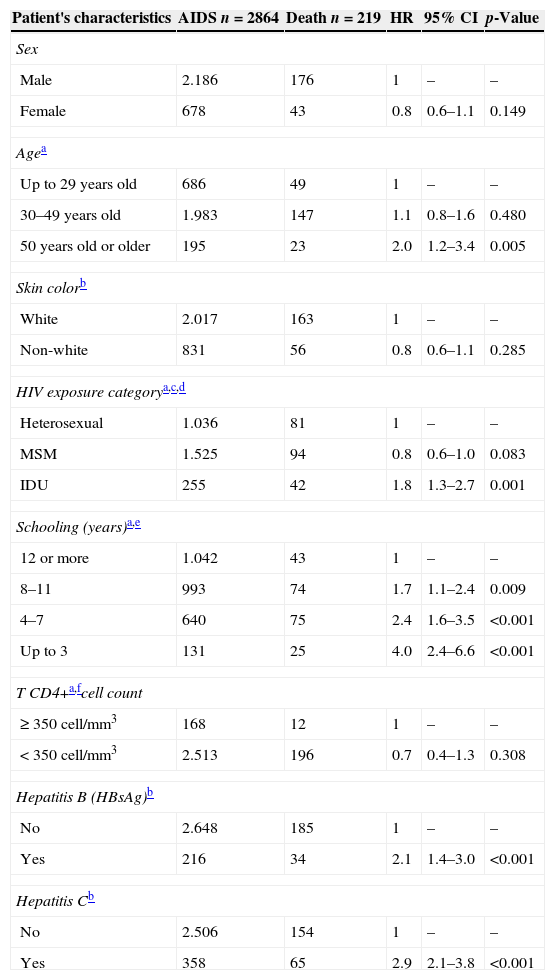
Results of Cox univariate analysis are presented in Table 2. HCV coinfection was a predictor of death (HR=2.9, CI 2.1–3.8). Other predictors of death were: Hepatitis B virus (HBV) coinfection (HR=2.1, CI 1.4–3.0), having up to three years of schooling (HR=4.0, CI 2.4–6.6), having four to seven years of schooling (HR=2.4, CI 1.6–3.5), having 8–11 years of schooling (HR=1.7, CI 1.1–2.4), IDU exposure category (HR=1.8, CI 1.3–2.7), and being 50 years or older (HR=2.0, CI 1.2–3.4).
Univariate Cox model analysis of predictive variables of death, CRT DST/AIDS-SP, 1986–2010.
| Patient's characteristics | AIDS n=2864 | Death n=219 | HR | 95% CI | p-Value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 2.186 | 176 | 1 | – | – |
| Female | 678 | 43 | 0.8 | 0.6–1.1 | 0.149 |
| Agea | |||||
| Up to 29 years old | 686 | 49 | 1 | – | – |
| 30–49 years old | 1.983 | 147 | 1.1 | 0.8–1.6 | 0.480 |
| 50 years old or older | 195 | 23 | 2.0 | 1.2–3.4 | 0.005 |
| Skin colorb | |||||
| White | 2.017 | 163 | 1 | – | – |
| Non-white | 831 | 56 | 0.8 | 0.6–1.1 | 0.285 |
| HIV exposure categorya,c,d | |||||
| Heterosexual | 1.036 | 81 | 1 | – | – |
| MSM | 1.525 | 94 | 0.8 | 0.6–1.0 | 0.083 |
| IDU | 255 | 42 | 1.8 | 1.3–2.7 | 0.001 |
| Schooling (years)a,e | |||||
| 12 or more | 1.042 | 43 | 1 | – | – |
| 8–11 | 993 | 74 | 1.7 | 1.1–2.4 | 0.009 |
| 4–7 | 640 | 75 | 2.4 | 1.6–3.5 | <0.001 |
| Up to 3 | 131 | 25 | 4.0 | 2.4–6.6 | <0.001 |
| T CD4+a,fcell count | |||||
| ≥ 350cell/mm3 | 168 | 12 | 1 | – | – |
| < 350cell/mm3 | 2.513 | 196 | 0.7 | 0.4–1.3 | 0.308 |
| Hepatitis B (HBsAg)b | |||||
| No | 2.648 | 185 | 1 | – | – |
| Yes | 216 | 34 | 2.1 | 1.4–3.0 | <0.001 |
| Hepatitis Cb | |||||
| No | 2.506 | 154 | 1 | – | – |
| Yes | 358 | 65 | 2.9 | 2.1–3.8 | <0.001 |
MSM, men who have sex with men; IDU, injecting drug users; HBsAg, hepatitis B surface antigen; HR, hazard ratio; CI, confidence interval.
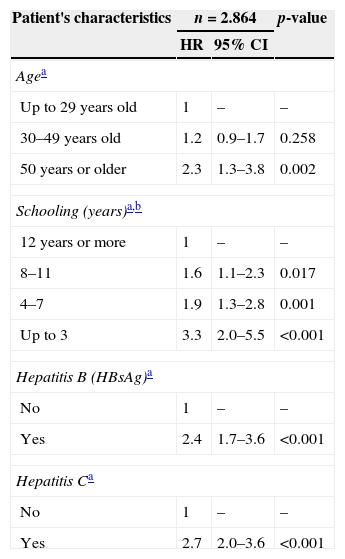
The final multivariate Cox regression model is presented in Table 3. The predictive variables of death, adjusted for other variables, were: HCV coinfection (HR=2.7, CI 2.0–3.6), HBV coinfection (HR=2.4, CI 1.7–3.6), having 8–11 years of schooling (HR=1.6, CI 1.1–2.3), having four to seven years of schooling (HR=1.9, CI 1.3–2.8), having up to three years of schooling (HR=3.3, CI 2.0–5.5), and being 50 years or older (HR=2.3, CI 1.3–3.8).
Multivariate Cox model analysis of predictors of death, CRT DST/AIDS-SP, 1986–2010.
| Patient's characteristics | n=2.864 | p-value | |
|---|---|---|---|
| HR | 95% CI | ||
| Agea | |||
| Up to 29 years old | 1 | – | – |
| 30–49 years old | 1.2 | 0.9–1.7 | 0.258 |
| 50 years or older | 2.3 | 1.3–3.8 | 0.002 |
| Schooling (years)a,b | |||
| 12 years or more | 1 | – | – |
| 8–11 | 1.6 | 1.1–2.3 | 0.017 |
| 4–7 | 1.9 | 1.3–2.8 | 0.001 |
| Up to 3 | 3.3 | 2.0–5.5 | <0.001 |
| Hepatitis B (HBsAg)a | |||
| No | 1 | – | – |
| Yes | 2.4 | 1.7–3.6 | <0.001 |
| Hepatitis Ca | |||
| No | 1 | – | – |
| Yes | 2.7 | 2.0–3.6 | <0.001 |
HR, hazard ratio; CI, confidence interval; HBsAg, hepatitis B surface antigen.
Concurrent infection by two or more agents is more harmful to human health.16
HIV–HCV coinfection was independently associated with increased risk of death in this study. Branch et al. have found 50% increased mortality among coinfected patients compared with non-coinfected.17 Although some other studies have not observed the same association,18 recent studies have consistently shown the burden of death among coinfected individuals.19–21
As already seen in other studies,4 the time of AIDS diagnosis was significantly associated with survival in our study. This is probably due to cART, which has changed the natural history of and clinical evolution of HIV infection and is available free-of-charge for Brazilian patients, distributed by the public health system since 1996.22
The survival curves in our study were significantly different between HCV–HIV coinfected and non-coinfected patients, a result similar to that of Bonacini et al.23 However, this difference was not significant for the periods of AIDS diagnosis between 2003 and 2010. This result can be explained by the immunosuppression control in patients undergoing cART24 and by the inclusion, in 2002, of pegylated interferon for treating hepatitis C in Brazil.25
In our study CD4+ T cell count was not associated with survival. Peters et al. have found that hepatitis C virus coinfection does not influence CD4+ T cell count recovery in HIV-1 infected patients with maximum virologic suppression.26 Nevertheless, by improving immune function, cART can slower the clinical evolution of HCV infection27 and reduce significantly the rate of deaths related to hepatitis C liver disease.28
The use of illicit injected drugs is known to be a risk factor for HCV infection. In our study, drug use was not independently associated with death, but this association was found by other authors.29
The prevalence of HCV infection has been estimated to have reached 10 million injected drug users worldwide, whereas 1.2 million would have been infected by HBV.30 The importance of this lies on the fact that cART is less beneficial in patients with coinfection, and adherence to therapy, which is already a problem in HIV–HCV coinfected patients,6 can be even lower among drug users.31,32 A meta-analysis published this year has shown that treatment of addiction results in higher hepatitis treatment completion including antivirals.33
Another coinfection significantly associated with increased risk of death in our study was HBV–HIV coinfection, as already reported by other authors.34 HIV–HBV coinfected individuals have accelerated hepatic fibrosis and reduced rates of spontaneous resolution of acute infection onset.35 But the influence of HBV infection in the course of AIDS is not known, and serological testing and vaccination are recommended to HIV infected individuals.
The association of increased survival and higher schooling came as no surprise in this study, as it had been already shown by others,36 as well as the association with advanced age,4,37,38 a finding which can be related to biological factors, social stigma, quantity and quality of social relationships.39,40 We did not find significant association of survival and gender and race in our study, but literature results are not consensual regarding this issue,41–43 and possibly it can be related to social factors such as access to care44,45 and adherence related to gender.42,43
The exclusion of dead patients who did not have HCV RNA results in the medical record and the exclusion of patients with late AIDS diagnosis (i.e. patients who died within 12 months from diagnosis) may have resulted in overestimation of survival rates and are limitations of this study.
ConclusionsAmong patients diagnosed after 1996, there was a significant increase in the cumulative probability of survival in HIV–HCV coinfected patients compared to previous years, and among those receiving AIDS diagnosis in the period from 2003 to 2010, this probability was similar between coinfected and non-coinfected patients, reflecting a possible impact of effective treatment of hepatitis C and cART on survival.
The results of our study are important and relevant for the clinical management and clinical policies designed for people living with HIV.
Conflicts of interestThe authors declare no conflicts of interest.
Study performed at Faculdade de Saúde Pública, Universidade de São Paulo.