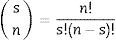Local epidemiological data are always helpful when choosing the best antibiotic regimen, but it is more complex than it seems as it may require the analysis of multiple combinations. The aim of this study was to demonstrate a simplified mathematical calculation to determine the most appropriate antibiotic combination in a scenario where monotherapy is doomed to failure.
MethodsThe susceptibility pattern of 11 antibiotics from 216 positive blood cultures from January 2012 to January 2013 was analyzed based on local policy. The length of hospitalization before bacteremia and the unit (ward or intensive care unit) were the analyzed variables. Bacteremia was classified as early, intermediate or late. The antibiotics were combined according to the combination model presented herein.
ResultsA total of 55 possible mathematical associations were found combining 2 by 2, 165 associations with 3 by 3 and 330 combinations with 4 by 4. In the intensive care unit, monotherapy never reached 80% of susceptibility. In the ward, only carbapenems covered more than 90% of early bacteremia. Only three drugs combined reached a susceptibility rate higher than 90% anywhere in the hospital. Several regimens using four drugs combined reached 100% of susceptibility.
ConclusionsAssociation of three drugs is necessary for adequate coverage of empirical treatment of bacteremia in both the intensive care unit and the ward.
Antibiotic therapy is essential for the proper treatment of infections.1 For community-acquired infections, protocols or consensus guidelines are extremely useful, since the susceptibility profile of bacteria may be quite similar in many areas of the world. Even when there are differences in antimicrobial susceptibility, administration of a large-spectrum antibiotic is sufficient to overcome this problem. In different hospitals, the ideal choice of antibiotics is hampered by extensive variability of bacteria and susceptibility profiles. Thus, the establishment of a consensus by the medical societies, even for regional entities, is rather challenging. Therefore, each hospital must assess its microbiological profile and propose treatment recommendations and protocols based on local data.
The knowledge of the local susceptibility profile can be used for choosing monotherapy. However, selecting the best antibiotic combination is far more complex since it involves dynamic calculations of the combination. Additionally, three to four drugs may be required to achieve an adequate coverage in high resistance scenarios, and this will generate hundreds of possible combinations.
The aim of this study was to demonstrate a simplified mathematical calculation to determine the most appropriate antibiotic association, considering dynamic but easily accessible epidemiological variables.
Materials and methodsA cross-sectional study was carried out at the Hospital Universitário Evangélico de Curitiba, a 660-bed tertiary-care university hospital in Curitiba, Brazil. This hospital is reference for renal transplantion, trauma and burn. All patients aged ≥18 years with a positive blood culture collected from January 2012 to January 2013 were included in the study. Only the first episode per patient was analyzed. Blood cultures yielding coagulase-negative Staphylococcus were considered contaminated and thus excluded. Data were collected from hospital computer system databases.
Blood cultures were collected according to the standard protocol used in the hospital and were processed using the BACT/Alert® (BioMerieux, Durham, USA). Bacteria were identified by Vitek 2 (Biomérieux, Marcy-L’Étoile, France). Susceptibility testing was performed using the disk diffusion method according to the CLSI guidelines.2 Molecular confirmation of extended and pan-resistant strains was routinely done.
The susceptibility pattern of 11 antibiotics was analyzed. The choice was based on local policy: aminoglycosides (amikacin and gentamycin), semi-synthetic penicillin with beta-lactamases inhibitor (ampicillin/sulbactam), third and fourth generation of cephalosporins (ceftriaxone, ceftazidime and cefepime), fluoroquinolones (ciprofloxacin and levofloxacin), ureidopenicillin with beta-lactamase inhibitor (piperacillin/tazobactam), glycopeptides (vancomycin and teicoplanin), polymyxin, carbapenems (meropenem and imipenem) and tetracyclines (tigecycline). For statistical analysis, aminoglycosides and carbapenems were considered to be resistant if any of the tested antibiotics in the same class was identified as resistant (e.g. if amikacin was susceptible and gentamycin resistant, the aminoglycoside group was considered resistant). Ceftazidime and ceftriaxone were evaluated separately. Tigecycline was included in the analysis once the incidence of KPC-producing Klebsiella pneumoniae was high in this hospital.3
The length of hospitalization before bacteremia and the unit (ward or intensive care unit – ICU) were the variables analyzed. Bacteremia was classified as early (<6 days), intermediate (6–14 days) and late (>14 days). This classification was created just for clinical purposes in the hospital.
For assessing combinations, the antibiotics were combined 2 by 2, 3 by 3, and 4 by 4 according to the following combination formula:
where “n” is the number of antibiotics and “s” the number of combined antibiotics.A large table was assembled to evaluate the percentage of antibiotic susceptibility for each bacterium to each group of antibiotic and for each combination of antibiotics (2 by 2, 3 by 3, and 4 by 4) (supplement table, http://infectopedia.com/dados-estatisticos/category/6-dados-estatisticos). When combinations were analyzed, the bacteria were considered susceptible for that combination if at least one antibiotic was active. The percentages in the table are the susceptibility rates against all bacteria during the period (100% for total susceptibility and 0% for no susceptibility).
Statistical analysisContinuous data are expressed as mean±standard deviation (SD) or median with ranges. Frequencies are expressed as percentages. Dichotomous variables were compared using χ2 test and Mann–Whitney test was used for continuous variables. Significance level was set at 0.05.
All data were recorded using the software Excel (Microsoft, New York, USA) and the statistical analysis was performed using the software SPSS 16 (SPSS, Chicago, USA). GraphPad Prism 5.0 (GraphPad, La Jolla, USA) was used for graphics.
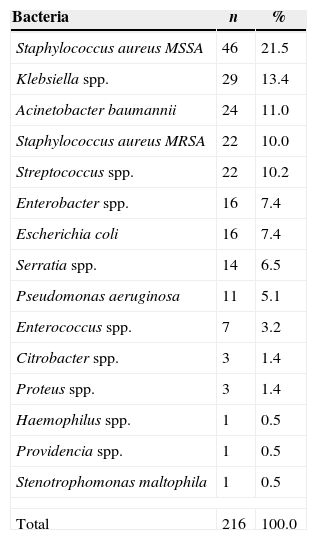
ResultsA total of 216 bacteremias were evaluated. Staphylococcus aureus was the most common bacteria found in the study (31.5%), followed by Klebsiella spp. (13.4%). The species distribution is detailed in Table 1. Early bacteremia (44.0%) was more frequent than late (34.7%) and intermediate (21.3%) (p<0.05). Bacteremia was more frequent in the ward (67.6%) than in the intensive care unit (32.4%) (p<0.05).
Bacterium species of 216 bacteremia in a hospital during January 2012 to January 2013.
| Bacteria | n | % |
|---|---|---|
| Staphylococcus aureus MSSA | 46 | 21.5 |
| Klebsiella spp. | 29 | 13.4 |
| Acinetobacter baumannii | 24 | 11.0 |
| Staphylococcus aureus MRSA | 22 | 10.0 |
| Streptococcus spp. | 22 | 10.2 |
| Enterobacter spp. | 16 | 7.4 |
| Escherichia coli | 16 | 7.4 |
| Serratia spp. | 14 | 6.5 |
| Pseudomonas aeruginosa | 11 | 5.1 |
| Enterococcus spp. | 7 | 3.2 |
| Citrobacter spp. | 3 | 1.4 |
| Proteus spp. | 3 | 1.4 |
| Haemophilus spp. | 1 | 0.5 |
| Providencia spp. | 1 | 0.5 |
| Stenotrophomonas maltophila | 1 | 0.5 |
| Total | 216 | 100.0 |
Using the combination formula, 55 associations were found combining 2 by 2, 165 associations with 3 by 3, and 330 combinations with 4 by 4. A total of 561 options for treatment were available.
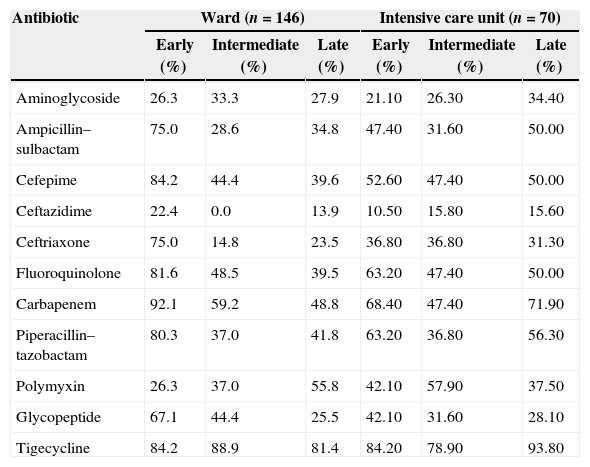
The susceptibility to antimicrobials with respect to the unit of admission and length of hospitalization is detailed in Table 2. In the ICU, no antibiotic reached 80% of susceptibility, with the exception of tigecycline (87%). This drug is not indicated for severely ill patients, and we excluded it from the analysis. Based on these data, one antibiotic is not enough as empirical treatment for bacteremia in the ICU setting. In the ward, early bacteremia showed better susceptibility pattern, with four antibiotics (cefepime, piperacillin/tazobactam, fluoroquinolone, and tigecycline) having more than 80–90%, and one (carbapenems) having 92% coverage. However, susceptibility rate was lower than 60% for all antibiotics (excluding tigecycline) in case of intermediate and late bacteremia.
Susceptibility pattern of each antibiotic for bacteria recovered from 216 bacteremia classified as early (< 5 days), intermediate (5 – 14 days) and late (>14 days). The bacteremia cases were categorized as from the intensive care unit or from the clinical ward.
| Antibiotic | Ward (n=146) | Intensive care unit (n=70) | ||||
|---|---|---|---|---|---|---|
| Early (%) | Intermediate (%) | Late (%) | Early (%) | Intermediate (%) | Late (%) | |
| Aminoglycoside | 26.3 | 33.3 | 27.9 | 21.10 | 26.30 | 34.40 |
| Ampicillin–sulbactam | 75.0 | 28.6 | 34.8 | 47.40 | 31.60 | 50.00 |
| Cefepime | 84.2 | 44.4 | 39.6 | 52.60 | 47.40 | 50.00 |
| Ceftazidime | 22.4 | 0.0 | 13.9 | 10.50 | 15.80 | 15.60 |
| Ceftriaxone | 75.0 | 14.8 | 23.5 | 36.80 | 36.80 | 31.30 |
| Fluoroquinolone | 81.6 | 48.5 | 39.5 | 63.20 | 47.40 | 50.00 |
| Carbapenem | 92.1 | 59.2 | 48.8 | 68.40 | 47.40 | 71.90 |
| Piperacillin–tazobactam | 80.3 | 37.0 | 41.8 | 63.20 | 36.80 | 56.30 |
| Polymyxin | 26.3 | 37.0 | 55.8 | 42.10 | 57.90 | 37.50 |
| Glycopeptide | 67.1 | 44.4 | 25.5 | 42.10 | 31.60 | 28.10 |
| Tigecycline | 84.2 | 88.9 | 81.4 | 84.20 | 78.90 | 93.80 |
Detailed rates of susceptibility for combined therapy are available as supplement material or at the site INFECTOPEDIA (http://infectopedia.com/dados-estatisticos/category/6-dados-estatisticos). In the table there are redundant antibiotic combinations such as cefepime with ceftriaxone. These combinations were included in the table just for statistical analysis, but must not be considered as clinical options for therapy.
In the ward, monotherapy for intermediate and late bacteremia had low coverage (up to 59% and up to 56%, respectively). Combination of two antibiotics reached a maximum of 85% susceptibility with carbapenem plus glycopeptide for intermediate bacteremia, and 83% for late bacteremia with carbapenem plus polymyxin. Only three drugs combined reached a coverage higher than 90%, using polymyxin plus glycopeptide associated with one of three options (cefepime, fluorquinolone or aminoglycoside).
For intermediate bacteremia in the ICU, two combined antibiotics reached a susceptibility rate of 89% for glycopeptide plus polymyxin, 89% for carbapenem plus polymyxin, and 95% for polymyxin plus fluoroquinolone. Tigecycline was not included in this analysis as it was not indicated for use in bacteremic patients. For late bacteremia, the same regimen using carbapenem plus polymyxin reached 91%, which increased to 97% by adding a glycopeptide. Several regimens using four drugs combined reached 100% susceptibility.
DiscussionThere are several publications showing the importance of an adequate antibiotic for the treatment of severe infections.4–7 Inadequate antibiotic therapy can increase mortality to a varying extent according to the population studied, severity of infection, clinical underlying conditions, and other epidemiological variables.8 However, other studies have not confirmed these findings, mainly in case of bacteremia in the ICU setting with comorbidities and advanced age.3,9,10
However, none of these studies defined the ideal rate of antibiotic coverage, regardless of the patient's clinical conditions. The question is: what should be the acceptable margin of error in the prescription of antibiotics for bacteremic patients? The ideal is 100% adequacy, but this figure can only be reached with at least three drugs, a suggestion not found in guidelines, including the IDSA guidelines for developing an institutional program to enhance antimicrobial stewardship.11
One could extensively discuss this issue. Several factors influence the decision of the physician when prescribing the antibiotic therapy, such as disease severity, patient age, comorbidities, organ dysfunction, previous colonization/infection, and probable site of infection. In some cases, it might be possible to delay the choice of antibiotics until the final result of cultures. However, in patients with severe sepsis, the decision must be taken in the first hours.12 Laboratory tests to identify bacteria and their susceptibility pattern to antibiotics in this window of time are not a reality yet.13 Nonetheless, when properly collected, culture results will allow the clinician to de-escalate all drugs to a broad spectrum specific therapy. De-escalation may be performed as soon as the result of Gram-positive blood culture is obtained, and it may be performed again after final identification.
Other questions about antibiotic prescription are: to determine if the patient is really infected; to consider candidemia or other fungi; how to de-escalation when blood cultures are negative; additional epidemiological variables may improve empirical decisions.
This simulation must be validated considering the following facts: (1) most cases of “fever” and laboratory alterations (mainly leukocytosis with immature cells) are not infection; (2) the infection is not severe, and we can wait for culture results; (3) the need for and effectiveness of antibiotic therapy in terminal patients; (4) side effects of extremely toxic associations (e.g. vancomycin, polymyxin, aminoglycoside); and (5) resistance induction and microbiote modification with further superinfection or Clostridium difficile colitis. Although combined therapy may be used for a short period, constant vigilance and follow up by an infectious diseases specialist is mandatory. Falagas et al. published an interesting article where they suppose that 50% of fever is not infection.14
The current results are the epidemiological panorama of a university hospital in a developing country, which cannot be applied to other health services and hospitals. The combination of three or four drugs can be used as a policy of antibiotic restriction. Carbapenems may be avoided with a regimen of ciprofloxacin+piperacillin/tazobactam. This can be an option in hospitals that practice antibiotic cycling.
Some bias probably may have occurred in this study, once patients with bacteremia in the ward had been previously admitted at the ICU, falsely increasing the rate of resistant in the ward.
The current study does not have the intention to convince clinicians to use bizarre and threatening antibiotic combinations, but rather to consider revision of current local guidelines and consider the mathematical formula of combination to construct an ideal regimen in that context. The same approach can be used for other infections, including urinary tract and respiratory infections, although one should consider cultures obtained from these sites. After this study, our hospital changed the antibiotic protocol for treating bacteremia, and other studies will be conducted in different sites. An internal validation must be performed followed by an external validation, if possible.
Antibiotic regimens with two, three or even four antibiotics seem so remote from what is currently considered to be good antibiotic prescribing practice that it may be difficult to be applied in clinical practice.
Conflicts of interestFelipe F. Tuon received grants from Bayer, Astellas, Merck, Pfizer, Novartis, AztraZeneca and United Medical (Gilead). Jaime L. Rocha received grants from Bayer, Merck, Pfizer, Novartis, Sanofi and AztraZeneca.