The aim this study was to determine the in vitro susceptibility to fosfomycin of bacteria isolated from urine samples of pregnant women with urinary tract infection. Samples of urine culture with bacterial growth of pregnant women were collected from clinical laboratories in Tubarão, state of Santa Catarina, Brazil, between September 2012 and May 2013. In the experimental stage, the colonies were tested for sensitivity to fosfomycin by using the Kirby–Bauer method. The following information relating to the samples was also collected: patients’ age, colony count, type(s) of identified bacterial(s) and result of the antimicrobial sensitivity test. Student's t-test was used for mean comparison. A total of 134 samples were selected for the study. The age of the subjects ranged from 15 to 40 years (mean 26.7). Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive) were the most commonly identified species. In 89% of cases, the microorganisms were sensitive to fosfomycin. E. coli and S. aureus were the main species of bacteria responsible for urinary tract infections in women in the study area. The most prevalent microorganisms in pregnant women with urinary tract infection were susceptible to fosfomycin.
Urinary tract infections (UTI) are common during pregnancy due to hormonal and anatomo-physiological changes that facilitate the growth and dissemination of bacteria in the maternal urinary tract.1 It is estimated that 5–10% of women develop some kind of UTI during pregnancy.2 Asymptomatic bacteriuria is the most common situation, followed by acute cystitis and pyelonephritis.3 These infections have potentially serious consequences for maternal and fetal health if they are not properly treated.1
The choice of antimicrobial agents should preferably consider the urinalysis results, which allow for pathogen identification, in addition to antimicrobial susceptibility testing that indicates the susceptibility of microorganisms to specific groups of antimicrobials.4 However, the correct choice of an antimicrobial agent to treat UTI during pregnancy is complex because it requires full attention to maternal and fetal safety, in addition to ease of use, access, and cost of treatment.5
Knowing the sensitivity of the etiological agents against antimicrobial drugs available, linked to relevant epidemiological data, helps to make appropriate therapeutic decisions based on updated scientific information for adequate treatment, which shortens the symptomatic period and prevents the possibility of recurrences and complications. However, in recent years, the problem of antimicrobial resistance has become very common, mainly because the antimicrobials once so successful, are no longer effective against several bacterial species responsible for infections in the urinary tract.3–6
Although the introduction rate of new classes of antibiotics on the market is limited, some old drugs such as fosfomycin can still provide a temporary solution to the emerging problem of resistance, since they have proven to be effective against the prevailing pathogens.
The aim of this study was to demonstrate the in vitro antibacterial susceptibility to fosfomycin of bacteria taken from urine samples of pregnant women with UTI.
This study was approved by the Research Ethics Committee of the University of Southern Santa Catarina in November 29, 2012 (code number 12.289.4.01.III).
The study was conducted in two stages: first, an epidemiological cross-sectional study to collect data related to urine cultures of pregnant women performed in laboratories of the city of Tubarão, Santa Catarina, Brazil, between September 2012 and May 2013; in the second stage, in vitro antibacterial susceptibility to fosfomycin of isolated bacterial colonies from the urinary tract of pregnant women was evaluated.
Thus, the samples consisted of positive urine cultures obtained from the participating laboratories, as well as the following information contained in the reports or patients’ records: colony count, identification of isolated species, antimicrobial susceptibility of the tested agents, and age of pregnant women.
The sample size was calculated according to the average annual number of live births in that city, which was 2470, and represented the estimated total number of annual pregnancies in the last decade (2001–2010), according to data from the Information System on Live Births.
The study sample was collected during nine months, and the final sample size of 186 pregnant women took into account the average annual number of pregnancies adjusted for the 9-month period (1853 pregnancies), and the percentage of UTI in pregnancy of 10%, as reported in the literature.2 For a confidence level of 95%, a minimum of 126 urine samples would have to be obtained.
The inclusion criteria included positive urine culture of pregnant women living in the city of Tubarão, Santa Catarina, according to Kass’ classification, regardless of symptoms. There was no access to clinical information regarding the patients’ signs and symptoms. Samples characterized by dehydrated cultures were excluded from the study because of possible contamination by non-pathogenic environmental fungi or other contaminant external agents. Cultures in which the necessary information was unavailable or inaccessible for any reason were excluded as well.
Information about the age of participating pregnant women, date of urine culture, and date of birth were retrieved from records and reports of the participating laboratories.
The selected samples were tested for sensitivity to fosfomycin by the disk diffusion method known as the Kirby–Bauer method, as recommended by the Clinical and Laboratory Standards Institute, described in document M100-S23, as of January 2013, and based on studies of Minimum Inhibitory Concentration. The disc used was SENSIBIODISC CECON Fosfomicina® for antibiogram in standard concentration of 200mcg of the drug, approved by the Brazilian Ministry of Health (Registration No. 10000600103) for testing Gram-positive and Gram-negative bacteria isolated from human urinary tract.
All data obtained in both stages of the study were organized and stored in Excel spreadsheet (Microsoft Office 2010) and analyzed with the use of descriptive statistics, and presented in a narrative, tabular, or graphical form. Statistical analysis was performed using the Statistical Product for Service Solutions v.20.0. Student's t test was used for mean comparison. The confidence level was set at 95%.
A total of 139 samples were selected for the study, of which five (3.6%) were excluded; three because of contamination and two due to lack of laboratory antimicrobial susceptibility testing results.
The age of participants ranged from 15 to 40 years (mean 26.7; SD 6.2). The most commonly identified microorganism was Escherichia coli (66.2%), followed by Staphylococcus aureus (14.3%), Klebsiella pneumoniae (3.8%), Enterobacter (3.0%), and others (12.7%).
The antimicrobial agents tested for antimicrobial sensitivity included nalidixic acid, ampicillin, sulphazotrim, ciprofloxacin, norfloxacin, and nitrofurantoin (Table 1).
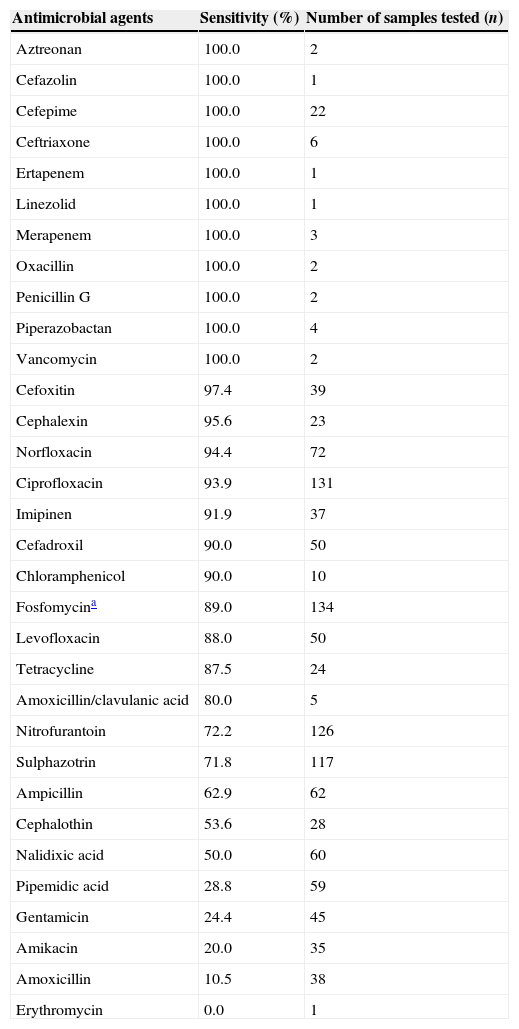
Results of antimicrobial susceptibility testing based on the sensitivity percentage and number of tested samples (n=134).
| Antimicrobial agents | Sensitivity (%) | Number of samples tested (n) |
|---|---|---|
| Aztreonan | 100.0 | 2 |
| Cefazolin | 100.0 | 1 |
| Cefepime | 100.0 | 22 |
| Ceftriaxone | 100.0 | 6 |
| Ertapenem | 100.0 | 1 |
| Linezolid | 100.0 | 1 |
| Merapenem | 100.0 | 3 |
| Oxacillin | 100.0 | 2 |
| Penicillin G | 100.0 | 2 |
| Piperazobactan | 100.0 | 4 |
| Vancomycin | 100.0 | 2 |
| Cefoxitin | 97.4 | 39 |
| Cephalexin | 95.6 | 23 |
| Norfloxacin | 94.4 | 72 |
| Ciprofloxacin | 93.9 | 131 |
| Imipinen | 91.9 | 37 |
| Cefadroxil | 90.0 | 50 |
| Chloramphenicol | 90.0 | 10 |
| Fosfomycina | 89.0 | 134 |
| Levofloxacin | 88.0 | 50 |
| Tetracycline | 87.5 | 24 |
| Amoxicillin/clavulanic acid | 80.0 | 5 |
| Nitrofurantoin | 72.2 | 126 |
| Sulphazotrin | 71.8 | 117 |
| Ampicillin | 62.9 | 62 |
| Cephalothin | 53.6 | 28 |
| Nalidixic acid | 50.0 | 60 |
| Pipemidic acid | 28.8 | 59 |
| Gentamicin | 24.4 | 45 |
| Amikacin | 20.0 | 35 |
| Amoxicillin | 10.5 | 38 |
| Erythromycin | 0.0 | 1 |
Eighty-nine percent of the microorganisms were sensitive to fosfomycin. Intermediate or resistant isolates included the strains of E. coli (5.9%; n=8), S. aureus (2.2%; n=3), Staphylococcus sp. (1.5%; n=2), Enterococcus sp. (0.7%; n=1), and Staphylococcus saprophyticus (0.7%, n=1).
The mean age of pregnant women with UTI caused by microorganisms sensitive to fosfomycin was 26.6 years (SD 6.2), whereas the mean age of those with UTI caused by resistant microorganisms was 27.2 years (SD 6.3), [p=0.731].
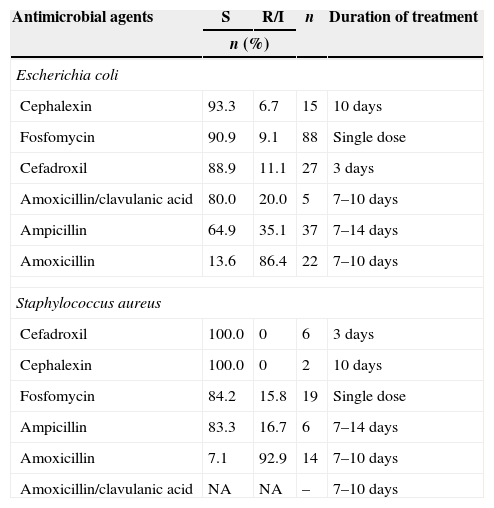
Relative sensitivity of the two most commonly isolated bacteria in this study to the orally administered antimicrobials safe for use during pregnancy is shown in Table 2.
Relative sensitivity profile of Escherichia coli and Staphylococcus aureus compared to the orally administered group of antimicrobial drugs safe for use during pregnancy.
| Antimicrobial agents | S | R/I | n | Duration of treatment |
|---|---|---|---|---|
| n (%) | ||||
| Escherichia coli | ||||
| Cephalexin | 93.3 | 6.7 | 15 | 10 days |
| Fosfomycin | 90.9 | 9.1 | 88 | Single dose |
| Cefadroxil | 88.9 | 11.1 | 27 | 3 days |
| Amoxicillin/clavulanic acid | 80.0 | 20.0 | 5 | 7–10 days |
| Ampicillin | 64.9 | 35.1 | 37 | 7–14 days |
| Amoxicillin | 13.6 | 86.4 | 22 | 7–10 days |
| Staphylococcus aureus | ||||
| Cefadroxil | 100.0 | 0 | 6 | 3 days |
| Cephalexin | 100.0 | 0 | 2 | 10 days |
| Fosfomycin | 84.2 | 15.8 | 19 | Single dose |
| Ampicillin | 83.3 | 16.7 | 6 | 7–14 days |
| Amoxicillin | 7.1 | 92.9 | 14 | 7–10 days |
| Amoxicillin/clavulanic acid | NA | NA | – | 7–10 days |
S, sensitive; R/I, resistant or intermediate.
E. coli was the most commonly identified microorganism, which supports the findings of other studies that indicate the predominance of this species in community-acquired UTIs among pregnant and non-pregnant women of different age-groups.2,4–7E. coli, in addition to be part of the normal intestinal microbial flora, is able to invade and remain in the urinary tract due to structural factors that facilitate adherence, which justify its predominance when compared to other species of the same family (Enterobacteriaceae), also causing UTI.8
Among the Gram-positive microorganisms, about 15% of the samples revealed the presence of S. aureus as the etiologic agent of UTIs. A study performed in a health center in Curitiba, Paraná that evaluated 120 urine samples of men and women with suspected UTI found a prevalence rate similar to that of this study, and S. aureus was isolated in 18.2% of cases.9 However, these findings differ from those of other studies in Portugal that found 0.9% of S. aureus in urine samples of uncomplicated cystitis in women, and 2.1% in urine samples of patients from a pathology service, which can be explained by the fact that S. aureus has been considered rare in community-acquired ITUs. In general, they are responsible for less than 4% of cases.10
Contradictory findings require clarification through new epidemiological studies in the geographic areas under scrutiny. This study did not aim to examine the criteria and methods for bacterial identification in the laboratories involved, which characterizes one of its limitations.
In this study, fosfomycin was effective in vitro useful against most strains and identified bacteria, which supports the findings of several other authors in similar studies11–13 and suggests a possible effectiveness in vivo. Andrade and collaborators have obtained 88% of UTI clinical cure among pregnant women through a single-dose oral administration of three grams of fosfomycin trometamol. They also found that the dose did not affect the development of fetuses and that adverse effects occurred in less than 10% of cases, limited to nausea or vomiting.14 In other clinical study, the treatment of cystitis in pregnant women with a single dose of fosfomycin trometamol was as effective as multiple doses. This medication is the preferred treatment of choice due to its ease of use.15
Fosfomycin has a bactericidal effect by inhibiting peptidoglycan synthesis and blocking the formation of N-acetylmuramic acid. Its wide spectrum of action reaches the target site in Gram-positive and Gram-negative bacteria.16 A single dose of fosfomycin is usually effective for the treatment of uncomplicated UTIs.17 Moreover, it is classified as a pregnancy category B drug by the Food and Drug Administration,3 which means that it is quite safe during pregnancy.
With regard to the performance of other antimicrobials, the analysis was focused on the most widely prescribed oral drugs for pregnant women, according to the literature. The most reliable medications include penicillin, cephalosporin and related compounds, which have low toxicity, even though they may cause unpredictable allergic reactions.18 Nitrofurantoin has been shown to be effective, but it is associated with hemolytic anemia of the newborn when used in the last trimester of gestation, although it is the most appropriate choice in the case of recurring infections.19 Quinolones are a pharmacological alternative, because they are classified as a pregnancy category B drug by the Food and Drug Administration, although its use is controversial because of the risk of damage to fetal cartilage and joint development.20,21 In this study, ciprofloxacin had a similar effect to that of fosfomycin. Contrastingly, the percentage of bacterial resistance to nitrofurantoin was almost one-third of the samples tested.
The present study found that amoxicillin associated with a clavulanic acid (beta-lactamase inhibitor) showed low performance for S. aureus, because the percentage of antibiotic-resistance was relatively high. Laboratories do not commonly test amoxicillin associated with beta-lactamase inhibitor, and this is a limiting factor for the conclusions of this study.
E. coli was resistant to amoxicillin, as well as to ampicillin, showing a high rate of resistance (above 20%). On the other hand, the sensitivity rate to cephalosporins and amoxicillin associated with clavulanic acid was equal to or above 80%. However, the number of samples tested for the latter was small, making the comparative analysis with fosfomycin less valid. It is noteworthy that the appearance of strains that produce beta-lactamases of an extended spectrum has increased among community- and nosocomial-acquired enterobacteria.22
It should be emphasized that none of the participating laboratories included fosfomycin on the list of antimicrobial agents for performing antimicrobial susceptibility testing of isolated bacteria. The results presented here were obtained in the evaluation phase of this study.
In the context of a rational use of antimicrobial agents, the drug choice has to take into account its efficacy, safety, convenience, and access. This means that the rational use of medications occurs when patients receive medications appropriate to their clinical needs, in doses that meet their own individual requirements, for an adequate period of time, and at the lowest cost to them and their community. In this sense, the inclusion of fosfomycin in antimicrobial susceptibility testing of urine cultures performed in clinical laboratories could predict whether it would be useful as a therapeutic option for UTI treatment, since this test shall guide the antibiotic therapy.
It should be noted that the study was focused on the information provided by participating laboratories without direct contact with pregnant women whose samples were included in the analyses. Therefore, further clinical data on patients, such as previous antibiotic use, gestational age, comorbidities, and in particular, the therapeutical procedures were not collected or analyzed. Those information items could be associated with clinical response and possible outcomes, such as relapse or reinfection. Thus, the effectiveness of fosfomycin in vivo could not be ultimately verified, with a better understanding of the interfering aspects and other elements related to individual situations.
The results of this study maintain fosfomycin as an option of choice for empiric use in cystitis among pregnant women, as recommended by the guidelines of the Brazilian Society of Infectious Diseases and the Brazilian Society of Urology. In most cases, empirical therapy is necessary to accelerate symptoms remission and prevent infection progression, at least while the urine culture and antimicrobial sensistivity testing are processed by the clinical laboratory. In Brazil, there is a paucity of research in antimicrobial resistance of urinary pathogens among adults in the community. Based on these results, new studies are warranted to define the prevalence of UTIs and clinical effectiveness of prescribed antibiotics.
In conclusion, out of the 136 analyzed isolates, E. coli and S. aureus were the main species responsible for UTIs among pregnant women in the study area. The most commonly used antimicrobial agents in the sensitivity tests performed by clinical laboratories included nalidixic acid, pipemidic acid, cefadroxil, nitrofurantoin, amoxicillin, ampicillin, sulphazotrim, ciprofloxacin, norfloxacin, and levofloxacin, many of which are contraindicated for pregnant women. Sensitivity of isolated bacteria to fosfomycin was 89%, which indicates that it might be an effective, safe and convenient therapeutic option for the treatment of uncomplicated UTIs during pregnancy.
Conflicts of interestThe authors declare no conflicts of interest.
We are thankful to the following clinical analysis laboratories of Tubarão, Santa Catarina: LAC/UNISUL, Santa Catarina, Santa Clara, Santé, Dr. Roberto and Cabral, and their respective administrative and technical staff, who voluntarily collaborated with this study, and made it possible.