The potential role of coffee as a hepatoprotective substance for chronic liver diseases has been widely discussed. Our main aim was to evaluate the effect of coffee intake regarding clinical, biochemical tests and liver biopsy data in treatment naïve patients with chronic hepatitis C. One hundred and thirty-six patients with chronic hepatitis C, diagnosed through liver biopsy, or by means of clinical, ultrasound or endoscopic signs of cirrhosis, were assessed by determination of biochemical tests, metabolic and morphological alterations. Food frequency was scrutinized by using a structured questionnaire. Coffee intake represented more than 90% of the total daily caffeine, and the 75th percentile was 4-Brazilian coffee-cup/day (≥255mL/day or ≥123mg caffeine/day). According to caffeine intake, patients were divided into two groups (< or ≥123mg caffeine/day). Patients with higher ingestion of caffeine had lower serum levels of aspartate aminotransferase (× upper limit of normal) (1.8±1.5 vs 2.3±1.5, p=0.04), lower frequencies of advanced (F3, F4) fibrosis (23.5% vs 54.5%, p<0.001) and of histological activity grade (A3, A4) observed in liver biopsies (13.8% vs 36.9%, p<0.001). By multivariate logistic regression, fibrosis was independently associated with caffeine intake (OR– 0.16; 95%CI – 0.03–0.80; p=0.026), γ-glutamil transferase serum levels and morphological activity. But only fibrosis was associated with histological activity. In conclusion caffeine consumption greater than 123mg/day was associated with reduced hepatic fibrosis. In addition, this study supports the assumption that coffee intake has hepatoprotective benefits for Brazilian patients with chronic hepatitis C, even in lower doses than that of American and European population intake.
Hepatitis C virus (HCV) infects chronically 130–170 million of the world's population.1 In Brazil, the prevalence of HCV infection ranges from 1 to 2%.2 Evidence suggests that caffeine may have hepatoprotective properties. In addition to caffeine, coffee contains chlorogenic acid, which has antioxidant and antimutation activities,3–5 and diterpenes (cafestol and kahweol) with anticarcinogenic properties.6
Coffee consumption, specifically with caffeine, has shown to be associated with a decreased risk of liver-associated enzyme elevations.7–9 Also, many studies have reported an inverse relationship between coffee drinking and the risk of liver cirrhosis.10–12 Some cohort13–15 and case-control studies,12,16–18 as well as two meta-analyses,19,20 suggested an inverse relationship between coffee drinking and the risk of hepatocellular carcinoma. Two studies showed that regular coffee intake (above a threshold of 308 or 408mg/day of caffeine) was associated with less severe hepatic fibrosis or with reduced histological activity in patients with chronic hepatitis C.21,22 Another recent study reported that higher coffee intake had a protective effect on non-alcoholic fatty liver disease23 and significant risk reduction for fibrosis among non-alcoholic steatohepatitis patients.24
Nowadays, the potential role of coffee as a hepatoprotective substance for chronic liver diseases has been widely discussed. Furthermore, the consumption of coffee by the Brazilian population has some peculiar characteristics in comparison with the European and American population. Thus, the main aim of this study was to evaluate the effect of coffee intake on clinical, biochemical and liver biopsy data in treatment naïve patients with chronic hepatitis C.
Materials and methodsPatientsOne hundred thirty-six treatment naïve patients with chronic hepatitis C were selected from the clinic of Gastroenterology Division Federal University of Sao Paulo, Escola Paulista de Medicina, between January 2009 and December 2011. They were enrolled into the study if they met the following criteria: positivity for serum HCV RNA; liver biopsy or diagnosis of hepatic cirrhosis based on clinical, ultrasound or endoscopic characteristics as well as the absence of previous antiviral therapy for chronic hepatitis C. Patients using drugs containing caffeine, co-infected with hepatitis B virus (serum HBsAg positive) or human immunodeficiency virus, excessive alcohol consumption (ethanol dose ≥20g/day for women and ≥40g/day for men), concomitant liver diseases, decompensated diabetes mellitus and clinically or biochemically recognized systemic diseases were excluded from this study.
The study was approved by the ethics committee of São Paulo Hospital of UNIFESP. All participants received detailed information about the study and gave written informed consent according to the norms of the Helsinki declaration.
Laboratory testsγ-Glutamil transferase (γGT), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) were determined by an automated kinetic method. Lipid profile (total cholesterol, high-density lipoprotein cholesterol (HDL-C) triglycerides, low-density lipoprotein cholesterol (LDL-C) and fasting glucose were measured by an automated colorimetric method. Insulin concentration was determined by immunofluorometric assay and insulin resistance was calculated by homeostasis model assessment (HOMA-IR) using the following formula: fasting insulin (μU/mL)×fasting glycemia (mmol/L)/22.5.25
Liver histopathologyAll liver biopsy specimens were fixed in formalin, embedded in paraffin and routinely processed for histological analysis. Histological scoring was performed according to the guidelines of the Brazilian Society of Pathology and Hepatology where the fibrosis score is similar to the Metavir classification, and inflammation semi-quantified as follows: grading of portal inflammation (0–4); interface hepatitis (0–4) and parenchymal necro-inflammatory lesions (0–4). The assessment of the inflammatory degree was done by the grade of interface hepatitis or with the sum of the portal inflammation, interface hepatitis and parenchymal lesion grade (histology activity index).26
Development of the caffeine questionnaireClinical interviews were performed by a dietitian using a structured questionnaire. A food frequency questionnaire was developed, using the format of the questionnaire used in the Health Professionals Follow-up Study Questionnaire,27 to evaluate caffeine intake. Patients were asked to quantify the frequency and the quantity of consumption of caffeine-containing products, including regular and diet carbonated soft drink beverages, regular coffee, decaffeinated coffee, black or green tea, cocoa/hot chocolate, caffeine-fortified drinks or chocolate candies. Consumption frequency was quantified as daily, weekly, monthly, occasionally or never. This questionnaire included data about caffeine-containing medications, coffee consumption time, the filtration method (paper or cloth), quantity of coffee used per month and the number of family members who drink coffee.
Statistical analysisTotal caffeine intake from foods and beverages (mg/day) was calculated by summing up caffeine content based on estimates from the published literature: regular coffee (137mg per 8-oz cup),22,28,29 decaffeinated coffee (2mg per 8-oz cup) (USDA), espresso coffee (64mg per 1-oz cup) (USDA), instant coffee (63mg per 8-oz cup) (USDA), green tea (30mg per 7-oz cup),30,31 chocolate candy bars (7.4mg per 30g).29 Daily caffeine intake for each patient was calculated from total coffee intake according to the frequency questionnaire. Caffeine intake was dichotomized above and below the 75th percentile according to the threshold for the cohort of the previous study.22
Chi-square or Fisher's exact test was performed for categorical variables, Mann–Whitney for continuous variables (non-parametric test) and “t” Student test (categorical variables, parametric test). Spearman's correlation was performed to evaluate the level of correlation between the variables studied. Univariate analysis and logistic regression were performed to identify independent predictors.
Statistical analyses were carried out using the SPSS 10.0 for Windows (SPSS, Inc., Chicago, IL, USA), with the level of significance set at p≤0.05.
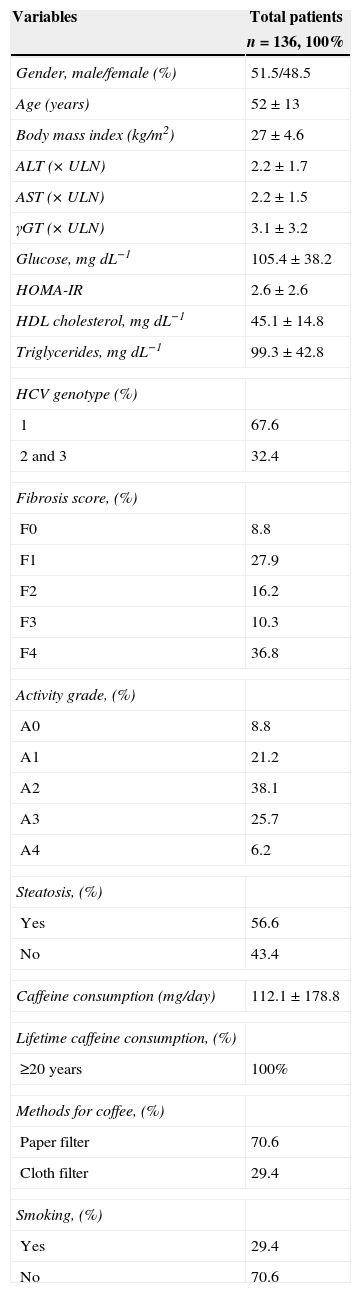
ResultsTable 1 shows baseline characteristics of the patients. Seventy patients were men (51.5%), the mean age of the overall group was 52±13 years and the mean body mass index (BMI) was 27±4.6kg/m2 and HCV genotype 1 (67.2%) was predominant. Of the 136 patients, 113 patients were biopsied and 23 patients were considered to have liver cirrhosis on the grounds of clinical and endoscopic and/or ultrasound examination. Activity grades ≥3 and 4 and fibrosis stage ≥3 and 4 were present in 47.1% and 31.9% of patients, respectively. The average estimated daily consumption of caffeine from foods and beverages was 115.5mg and of all the caffeine consumed, 96.6% came from regular coffee, 2.8% from caffeinated soft drinks, 0.4% from chocolate and 0.2% from tea.
Baseline characteristics of the patients.
| Variables | Total patients |
|---|---|
| n=136, 100% | |
| Gender, male/female (%) | 51.5/48.5 |
| Age (years) | 52±13 |
| Body mass index (kg/m2) | 27±4.6 |
| ALT (×ULN) | 2.2±1.7 |
| AST (×ULN) | 2.2±1.5 |
| γGT (×ULN) | 3.1±3.2 |
| Glucose, mgdL−1 | 105.4±38.2 |
| HOMA-IR | 2.6±2.6 |
| HDL cholesterol, mgdL−1 | 45.1±14.8 |
| Triglycerides, mgdL−1 | 99.3±42.8 |
| HCV genotype (%) | |
| 1 | 67.6 |
| 2 and 3 | 32.4 |
| Fibrosis score, (%) | |
| F0 | 8.8 |
| F1 | 27.9 |
| F2 | 16.2 |
| F3 | 10.3 |
| F4 | 36.8 |
| Activity grade, (%) | |
| A0 | 8.8 |
| A1 | 21.2 |
| A2 | 38.1 |
| A3 | 25.7 |
| A4 | 6.2 |
| Steatosis, (%) | |
| Yes | 56.6 |
| No | 43.4 |
| Caffeine consumption (mg/day) | 112.1±178.8 |
| Lifetime caffeine consumption, (%) | |
| ≥20 years | 100% |
| Methods for coffee, (%) | |
| Paper filter | 70.6 |
| Cloth filter | 29.4 |
| Smoking, (%) | |
| Yes | 29.4 |
| No | 70.6 |
Continuous data are expressed as mean±standard deviation and categoric data are expressed as percentage. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; (GT, (-glutamyl transferase; HOMA-IR, homeostasis model assessment of insulin resistance (fasting insulin [(U/mL]×fasting glycemia [nmol/L]/22.5); HDL cholesterol, high-density lipoprotein cholesterol; ULN, upper limit of normal.
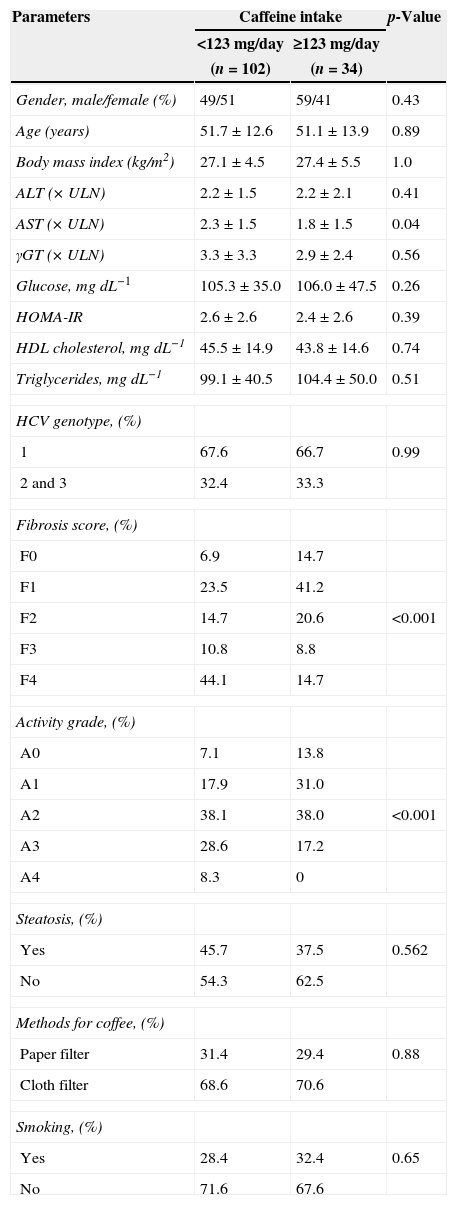
The patients were divided into groups, according to the 75th percentile (123mg of caffeine) (Table 2). The two groups did not present significant differences in demographic or anthropometric characteristics. However, significant reduction in AST serum level (×ULN) (1.8±1.5 vs 2.3±1.5, p=0.04) was observed in the group with high caffeine ingestion. Caffeine intake related to greater than 4-Brazilian coffee-cup/day (≥255mL/day or ≥123mg caffeine/day) was also associated with lower frequencies of advanced fibrosis (F3, F4) (23.5% vs 54.5%, p<0.001) and histological activity grade (A3, A4) observed in liver biopsies (13.8% vs 36.9%, p<0.001) (Table 2).
Characteristics of the patients according to caffeine intake.
| Parameters | Caffeine intake | p-Value | |
|---|---|---|---|
| <123mg/day | ≥123mg/day | ||
| (n=102) | (n=34) | ||
| Gender, male/female (%) | 49/51 | 59/41 | 0.43 |
| Age (years) | 51.7±12.6 | 51.1±13.9 | 0.89 |
| Body mass index (kg/m2) | 27.1±4.5 | 27.4±5.5 | 1.0 |
| ALT (×ULN) | 2.2±1.5 | 2.2±2.1 | 0.41 |
| AST (×ULN) | 2.3±1.5 | 1.8±1.5 | 0.04 |
| γGT (×ULN) | 3.3±3.3 | 2.9±2.4 | 0.56 |
| Glucose, mgdL−1 | 105.3±35.0 | 106.0±47.5 | 0.26 |
| HOMA-IR | 2.6±2.6 | 2.4±2.6 | 0.39 |
| HDL cholesterol, mgdL−1 | 45.5±14.9 | 43.8±14.6 | 0.74 |
| Triglycerides, mgdL−1 | 99.1±40.5 | 104.4±50.0 | 0.51 |
| HCV genotype, (%) | |||
| 1 | 67.6 | 66.7 | 0.99 |
| 2 and 3 | 32.4 | 33.3 | |
| Fibrosis score, (%) | |||
| F0 | 6.9 | 14.7 | |
| F1 | 23.5 | 41.2 | |
| F2 | 14.7 | 20.6 | <0.001 |
| F3 | 10.8 | 8.8 | |
| F4 | 44.1 | 14.7 | |
| Activity grade, (%) | |||
| A0 | 7.1 | 13.8 | |
| A1 | 17.9 | 31.0 | |
| A2 | 38.1 | 38.0 | <0.001 |
| A3 | 28.6 | 17.2 | |
| A4 | 8.3 | 0 | |
| Steatosis, (%) | |||
| Yes | 45.7 | 37.5 | 0.562 |
| No | 54.3 | 62.5 | |
| Methods for coffee, (%) | |||
| Paper filter | 31.4 | 29.4 | 0.88 |
| Cloth filter | 68.6 | 70.6 | |
| Smoking, (%) | |||
| Yes | 28.4 | 32.4 | 0.65 |
| No | 71.6 | 67.6 | |
Continuous data are expressed as mean±standard deviation and categoric data are expressed as percentage. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; γGT, γ-glutamyl transferase; HOMA-IR, homeostasis model assessment of insulin resistance (fasting insulin [μU/mL]×fasting glycemia [nmol/L]/22.5); HDL cholesterol, high-density lipoprotein cholesterol; ULN, upper limit of normal.
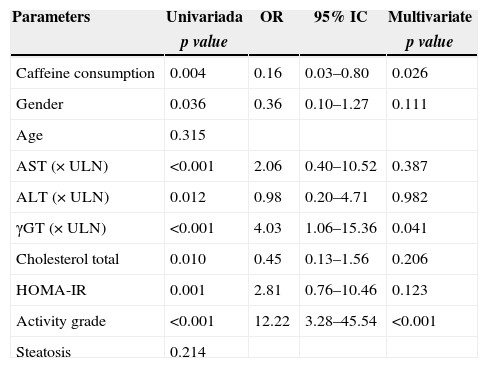
To clarify the effect of the caffeine intake on liver fibrosis and histological activity in the hepatitis C evolution, both univariate analysis and logistic regression were performed. In univariate analysis, advanced fibrosis (fibrosis stage ≥3 and 4) was significantly associated to caffeine intake (p=0.004), gender (p=0.036), AST serum level (p<0.001), ALT serum level (p=0.012), γGT serum level (p<0.001), total cholesterol serum level (p=0.01), HOMA-IR (p=0.001) and histological activity (p<0.001). By multivariate logistic regression, advanced fibrosis was independently associated with caffeine intake (OR=0.16; 95% CI: 0.03–0.80; p=0.026); γGT serum level (OR=4.03; CI 95%: 1.06–15.36; p=0.041) and histological activity (OR=12.22; CI 95%: 3.28–45.54; p<0.001) (Table 3).
Univariate and logist regression analysis of factors associated with fibrosis.
| Parameters | Univariada | OR | 95% IC | Multivariate |
|---|---|---|---|---|
| p value | p value | |||
| Caffeine consumption | 0.004 | 0.16 | 0.03–0.80 | 0.026 |
| Gender | 0.036 | 0.36 | 0.10–1.27 | 0.111 |
| Age | 0.315 | |||
| AST (×ULN) | <0.001 | 2.06 | 0.40–10.52 | 0.387 |
| ALT (×ULN) | 0.012 | 0.98 | 0.20–4.71 | 0.982 |
| γGT (×ULN) | <0.001 | 4.03 | 1.06–15.36 | 0.041 |
| Cholesterol total | 0.010 | 0.45 | 0.13–1.56 | 0.206 |
| HOMA-IR | 0.001 | 2.81 | 0.76–10.46 | 0.123 |
| Activity grade | <0.001 | 12.22 | 3.28–45.54 | <0.001 |
| Steatosis | 0.214 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; γGT, γ-glutamyl transferase; HOMA-IR, homeostasis model assessment of insulin resistance (fasting insulin [μU/mL]×fasting glycemia [nmol/L]/22.5); ULN, upper limit of normal.
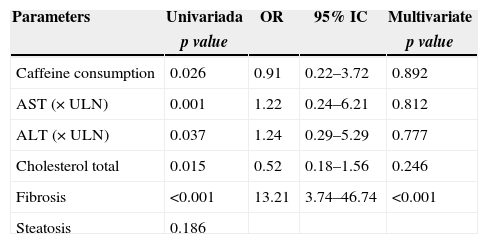
A similar analysis for advanced histological activity grade observed in liver biopsies (activity grades ≥3 and 4) was performed. By univariate analysis, severe histological activity grade was significantly associated to caffeine intake (p=0.026), AST serum level (p=0.001), ALT serum level (p=0.037), total cholesterol serum level (p=0.015) and liver fibrosis (p<0.001). However, by multivariate logistic regression, only liver fibrosis was associated with histological activity grade. No association could be found between histological activity and caffeine intake (Table 4).
Univariate and logistic regression analysis of factors associated with activity grade.
| Parameters | Univariada | OR | 95% IC | Multivariate |
|---|---|---|---|---|
| p value | p value | |||
| Caffeine consumption | 0.026 | 0.91 | 0.22–3.72 | 0.892 |
| AST (×ULN) | 0.001 | 1.22 | 0.24–6.21 | 0.812 |
| ALT (×ULN) | 0.037 | 1.24 | 0.29–5.29 | 0.777 |
| Cholesterol total | 0.015 | 0.52 | 0.18–1.56 | 0.246 |
| Fibrosis | <0.001 | 13.21 | 3.74–46.74 | <0.001 |
| Steatosis | 0.186 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; γGT, γ-glutamyl transferase; ULN, upper limit of normal.
With the threshold of the 75th percentile to identify patients with greater caffeine intake, we found that these HCV patients presented lower levels of serum AST levels and lower frequency of advanced inflammatory activity and fibrosis degree when compared with those with lower caffeine intake. These findings are consistent with previous studies suggesting a liver protective effect of caffeine in patients with other liver diseases.8,9,21,22,32,33 In order to assess the impact of caffeine ingestion on histological parameters, univariate and multivariate logistic regression were performed with the degree of fibrosis as a continuous variable, in order to clarify the real impact of caffeine in liver disease progression. In such an analysis, daily caffeine intake higher than 123mg (4-Brazilian coffee-cup/day) stood out as an independent predictor of a lower risk of advanced fibrosis, together with serum γGT level and histological activity, two parameters previously described as independent risk factors for fibrosis. Although significant in univariate analysis, caffeine ingestion could not be associated with inflammatory activity, assessed by interface hepatitis or even by the histology activity index (data not shown), by logistic regression. It is possible that the exclusion of 17% of the patients from the cohort (those with overt cirrhosis and no liver biopsy performed) had reduced the strength of the analysis for the association between activity and caffeine intake, but the association between fibrosis degree and daily caffeine ingestion persisted even after the exclusion of these patients with overt cirrhosis.
Comparing this study with those previously published, it became clear that there is a difference between the pattern of daily caffeine ingestion between Brazilian, European and American patients with HCV chronic infection. Coffee intake accounts for more than 95% of the daily caffeine ingestion in our cohort while it has been reported to be around 70% among American patients; in this study, the threshold of the 75th percentile was ≥123mg caffeine/day (≥255mL/day or 4-Brazilian coffee-cup/day), while other studies report a cut-off of 308mg caffeine/day22 or 407mg caffeine/day.21 These differences allowed us to demonstrate that a lower cut-off of caffeine intake has liver protective benefits. Protective effect of caffeine on liver was significant and evaluated as a continuous variable, categorized as coffee-cup equivalents, or dichotomized above or below the 75th percentile for the study population. Categorization of caffeine intake by coffee-cup equivalents or quartile suggested that the protective effect of caffeine may not be linear, and there appears to be a threshold effect. Approximately 4-Brazilian coffee-cup/day, was necessary to have an effect on fibrosis progression in these patients with chronic hepatitis C. Clarification of whether there is a hepatoprotective threshold and whether the benefits eventually plateau with further consumption will be important for understanding the biology and potentially for therapeutic recommendations. Furthermore, these results strongly support the assumption that coffee's caffeine has hepatoprotective benefits in Brazilian patients with chronic hepatitis C, since, in this cohort, coffee was almost the only source of caffeine intake.
Coffee contains a variety of chemical compounds, including caffeine, chlorogenic acid, quinides, trigonelline and lignin. The standardization of these compounds’ concentration in a cup of coffee is difficult and the food frequency questionnaires are useful tools, but are subject to misclassification. To our knowledge, this is the first study able to examine the preparation and serving methods of coffee. For this reason, coffeine consumption via the number of consumed cups of coffee may correctly reflect the differences between the groups. Analyzing methods used to collect dietary data on coffee and caffeine intake in previous studies,21,22,32,34 we observed that several authors had used only the food frequency questionnaire, which was self-administered or applied by the health-professional nurse. In the present study, in order to further investigate the dietary intake of coffee and caffeine, 24-h dietary recall was associated with a food frequency questionnaire and both applied by nutritionists. In professional practice, nutritionists are able to develop specific actions.35 The food frequency questionnaire is the most widely used in population studies and more appropriate to classify individuals according to intake of foods or nutrients.29
Here, patients with higher caffeine intake had also lower AST levels. Similarly, a study enrolling Japanese patients with no previous liver dysfunction reported that coffee intake was inversely associated to AST and ALT serum concentrations, regardless of age, BMI, alcohol intake and smoking.7 Another study identified lower ALT levels in patients with higher caffeine intake and it suggested that caffeine has an antioxidant inhibiting lipid peroxidation role and consequently, the formation of free radicals.8 Previous studies have shown that increased coffee consumption is associated with lower liver enzymes, reduced rates of liver cancer, and possibly even reduced hepatic decompensation and liver-related mortality.8,9,11,33,35
The mechanisms underlying potential hepatoprotective effects of caffeine in patients with chronic hepatitis C remain to be determined. Caffeine is a purine alkaloid, acting through the antagonism of adenosine receptors A1 and A2.23 Recent studies have demonstrated that adenosine, acting at A2 receptors, stimulates hepatic stellate cell-mediated fibrosis of the liver29,36–39 by increasing production of collagen I and III (the collagens present in scar tissue) via two distinct mitogen-activated protein kinase (MAPK)-dependent pathways, extracellular signal-regulated kinase 1/2 (ERK1/2) and p38MAPK, respectively.40 Interestingly, caffeine, the most widely used drug in the world, mediates most of its pharmacological effects by non-selectively blocking adenosine receptors, including A2 receptors, and can prevent hepatic fibrosis in animal models.41,42 Several reports suggest caffeine and other constituents of coffee, as Kahweol and cafesterol, possess antioxidant properties.43–45 Studies in mice and rats as well as with human hepatoma cell lines have shown that coffee and some of its major components (caffeine, cafestol, and kahweol) alter expression and activity of enzymes involved in xenobiotic metabolisms.46,47 Inhibition of phase I enzymes and up-regulation of phase II enzymes such as glutathione-S-transferase have been reported, both of which would favor reduced accumulation of toxic metabolites within hepatocytes.46 Pre-treatment with cafestol and kahweol protected mice from carbon tetrachloride hepatotoxicity by inhibiting cytochrome CYP 2E1, the enzyme responsible for carbon tetrachloride bioactivation.48 With respect to caffeine specifically, a study reported that caffeine inhibits expression of a connective tissue growth factor (CTGF) by interfering with the transforming growth factor beta (TGFβ) signaling through the similar to mothers against decapentaplegic in drosophila (SMAD) pathway.49 Caffeine was also found to up-regulate peroxisome proliferator-activated receptor gamma (PPARγ) levels, which further reduce CTGF expression. Although these results from primary cell culture clearly need in vivo confirmation, inhibition of the transforming growth factor beta pathway is an attractive explanation for anti-fibrogenic effects attributed to caffeine.22
In conclusion, a useful tool for a comprehensive evaluation of caffeine intake was applied and it indicated a beneficial effect, which requires caffeine intake above a threshold of approximately 4-Brazilian coffee-cup/day. In addition, our study showed that coffee intake has hepatoprotective benefits - an inverse association of the increased caffeine intake with a lower risk of advanced fibrosis - in treatment naïve Brazilian patients with chronic hepatitis C even in different doses from American and European intake.
Conflict of interestThe authors declare no conflicts of interest.