Screening for vertically transmitted infection is mandatory and must be conducted at the first prenatal consultation. The most vulnerable women's groups are those at the lowest socio-economic level. Dried blood spot testing on filter paper could represent a secure way to screen pregnant women in the prenatal period.
MethodsA cross-sectional study was conducted between November 2009 and March 2010, in the Metropolitan Region of Salvador, Bahia, Brazil, to compare the accuracy of the dried blood spot in filter paper and venipuncture serological as screening methods for HIV, HTLV, VHB, VHC, Treponema pallidum, and Toxoplasma gondii during prenatal period. Results of the venous blood sample collected in tubes were considered the gold standard.
ResultsSerum samples and dried blood spot were obtained from 692 pregnant women aged between 14 and 42 years, with a median age of 26. Thirteen women were seropositive for T. gondii (1.88%; 95% CI: 0.60–2.71%), five for T. pallidum (0.72%; 95% CI: 0.15–1.61%), two for HBV (0.29%; 95% CI: 0.050.95%) and one for HTLV-1 (0.14%; 95% CI: 0.01–0.71%). No one was positive for HCV and HIV. The dried blood spot accuracy for syphilis and HTLV were 100% (95% CI: 99.25–100) and 100% (95% CI: 99.45–100%), respectively. The average time between blood collection and recording of the sample in the reference laboratory was 4.93 (3.82) days and between dried blood spot processing and active search for pregnant women was 3.44 (4.27) days.
ConclusionsThe use of dried blood spot may represent a secure way to expedite access to results of vertically transmitted diseases in the prenatal period, particularly in regions with scarce healthcare resources.
Implementation of the Prenatal and Birth Humanization Programme (Programa de Humanização do Pré-Natal e do Nascimento: PHPN) of the Brazilian Ministry of Health has established objective standards to assess the quality of prenatal care.1 These criteria include the percentage of pregnant women who undergo anti-Human Immunodeficiency Virus (HIV) test and syphilis serology test in both the 1st and 3rd trimesters of pregnancy.1,2
In Brazil, serological screening for HIV in pregnant women is recommended and should be conducted at the first prenatal consultation.3 However, weaknesses in healthcare network provision of laboratory diagnosis, insufficient coverage of women tested in the prenatal period hinder adequate prophylaxis to approximately 50% of pregnant women infected with HIV.3 This specially is the case for women most vulnerable to HIV such as sex workers, injecting drug users, and those of lower socio-economic strata,4 as a result of low quality of prenatal care.5 Likewise, the same is observed for other infections such as syphilis, hepatitis B and C and toxoplasmosis, causing diseases in pregnancy and fetal morbidity and mortality.2,5,6 Diagnosis of human T-cell lymphotropic virus-type 1 (HTLV-1) infection is also frequently missed despite being prevalent in Brazil, predominantly in the North and Northeast regions.7,8
Despite such recommendations, both the supply of and access to serology during the prenatal period remain highly restricted.4,9 Furthermore, qualitative data state that two factors related to greater adherence to prenatal care are availability of tests within primary healthcare units, associated with good quality professional care.10 Thus, procedures that increase access to serologic diagnosis and guarantee that the pregnant woman receives her results within the required time must be implemented within the Network of Primary Healthcare Units (Unidades da Rede Básica de Saúde: UBS) and at Family Health Programme (Programa de Saúde da Família: PSF) centers. Dried blood spot testing on filter paper (DBS), as a vehicle for the storage and transportation of blood, has been proven successful in neonatal screening11 and has some advantages in relation to traditional plasma and serum samples, such as ease of collection, transportation and storage, room temperature sample stability, and less risk of contamination.12
The use of DBS as a screening tool to detect HIV antibodies has occurred in epidemiological surveys since the beginning of the 1990s,13 and is considered an option in both developing countries and remote regions where appropriate laboratory support does not exist.12–14 In Brazil, this strategy for the identification of infectious agents has been assessed as successful,15–18 including for prenatal screening.19–21
Although several studies have demonstrated the accuracy of serological screening for HIV, HTLV and other infectious agents when comparing DBS with plasma and serum,16,17 few validation studies have been conducted in the primary healthcare network22,23 and no studies in our environment have measured the operational time elapsed between blood collection and availability of the result.
In this work we have assessed the prevalence of HIV, HTLV, VHB, VHC, syphilis and toxoplasmosis in pregnant women; the accuracy of serological screening using DBS compared to serum; and the time elapsed between blood collection, laboratory processing and result availability at the primary healthcare coordination department of the Unitary Health System (Sistema Único de Saúde: SUS) in the municipality of Lauro de Freitas, in the Metropolitan Region of Salvador, Bahia.
Materials and methodsStudy design and locationA cross-sectional study was conducted between November 2009 and March 2010 to evaluate the accuracy of DSB compared to standard serological screening methods for HIV, HTLV, VHB, VHC, Treponema pallidum (T. pallidum), and Toxoplasma gondii (T. gondii).
The study was conducted in Lauro de Freitas, in the Metropolitan Region of Salvador (Região Metropolitana de Salvador: RMS), the state capital of Bahia, in North East Brazil. In 2010, this municipality had 163,449 inhabitants and, in terms of gross domestic product (GDP), was ranked 25th in the North East region, with approximately 0.6% of the region's total GDP. In 2009, 22.3% of the RMS population had private health insurance.a There were 84,173 (51.5%) female inhabitants, of which 57,854 were of childbearing age (10–49 years old), accounting for 68.7% of the municipality's female population, with an average monthly income of R$1217.00 (US$ 546.69), with a ratio of 0.64 in relation to the average salary of men. In approximately 40% the household head were the women.
In January 2010, the municipality had 16 Primary Healthcare Units (UBS) distributed across seven administrative sub-units. The following units were enrolled in the study: Centre for Women's Health, Citizen's Space, São Paulo Park UBS, Cidade Nova UBS, São Judas Tadeu UBS, Israel Moreira UBS, Outpatient's Clinics in the Centre and in Caji-Vida Nova, Irmã Dulce UBS, Vila Nova UBS and Noel Alves da Cruz UBS. These units were in locations that provide prenatal care or offer pregnant women serological testing. The district of Areia Branca was not included because of its small representative population, distance from the municipal center and since it does not provide laboratory services related to the collection of biological material for serological testing.
Routines for healthcare and the collection of biological materialDuring the study period, all pregnant women who spontaneously attended the prenatal healthcare network and SUS's own- and affiliated-laboratory networks in order to carry out the required prenatal care tests at the appropriate units were invited to take part. Following an explanation of the study objectives, risks and expected benefits and after signing the Free and Informed Consent Form, about 10mL of blood was obtained by venipuncture and approximately 9.0mL of the material was packed in a drying tube, while the rest was packed on Schleicher & Schuell 903© filter paper in five circles of 1.0cm diameter.24 We did not collect a new filter paper sample from the women who had already conducted the serum and paper filter test during the study period, but followed the prenatal care routine, carrying out the VDRL test, as recommended by the Ministry of Health.9
The DBS sample was identified through the woman's name, date of birth and collection, date of last menstruation, gestational age, past obstetric history (number of pregnancies, abortions and births) and healthcare unit of collection. All the drying, packing and storage procedures for the DBS paper filters were conducted as previously described.22 At the end of the daily collections, the DBS and blood collection tubes were dispatched to the Central Laboratory (Laboratório Central: LACEN) of Lauro de Freitas to be stored in a refrigerator. In addition, we dispatched the DBS to the Neonatal Screening Laboratory of the Association of Parents and Friends of Handicapped Children of Salvador (Associação de Pais e Amigos dos Excepcionais de Salvador: APAE Salvador) in accordance with the municipal administrative routine, previously instituted for the “heel prick test”.
Elapsed time between collection and registration of the sample at APAE Salvador was called sample retention time and elapsed time between arrival of the sample at the laboratory and release and immediate communication of the result to the Municipal Health Department (Secretaria Municipal de Saúde: SMS) was called active search time. Specialized care for pregnant woman with HTLV was available at the HTLV Centre at the Bahian School of Medicine and Public Health (Escola Bahiana de Medicina e Saúde Pública: EBMSP) and for the other women tested this was available at the municipality's reference units for the care of STDs/AIDS.
Laboratory methodsThe samples were processed to detect anti-HIV 1+2, anti-HTLV-1 and 2, anti-VHC antibodies, anti T. pallidum (IgM and IgG), anti-T. gondii IgM antibodies and HBsAg on filter paper and plasma using the enzyme linked immunosorbent assay (ELISA). The immunological markers (antibodies) were obtained by an elution process, using a buffered solution containing protein, detergent, stabilizers and 0.1% sodium azide, and then subjected to ELISA, as recommended by the manufacturer. The serology tests were blindly performed on eluates obtained from the filter paper and serum at the Laboratory of the Centre for Diagnosis and Research (Laboratório do Centro de Diagnóstico e Pesquisa: CEDIP) of APAE Salvador, according to its laboratory routine.
We used the following commercial kits on the material obtained on filter paper.
IMUNOSCREEN HIV 1+2 – SS©, IMUNOSCREEN HTLV I and II – SS©, IMUNOSCREEN ANTI-HCV – SS©, IMUNOSCREEN TOXO IGM – SS©, IMUNOSCREEN SÍFILIS – SS© and IMUNOSCREEN HBSAG – SS©, produced by MBIOLOG Diagnósticos LTD, Contagem, MG, Brazil,26 which, at the time of the study, was the only manufacturer in our country that produced diagnostic paper filter kits for the studied agents. We used the following commercial kits for the samples obtained in serum: MUREX HIV I and II©, MUREX HTLV I and II©, MUREX ANTI-HCV©, MUREX ICE SYPHILIS©, BIOELISA TOXO IgM©, MUREX HBsAg©, produced by MUREX BIOTECH LIMITED, Dartford, Kent, United Kingdom.
Statistical analysisThe prevalence rates were calculated and the 95% confidence intervals (95% CI) reported for each surrogate marker of the infections studied. Sensitivity and specificity for each infection marker were computed with their respective 95% CI. A database was constructed in Epi Info for Windows© version 3.2.2 and calculations were made using the OpenEpi programme, version 2.1.3.25 For continuous variables averages, medians and standard deviations (SD) are reported.
Ethical aspectsThe study was approved by the Research Ethics Committee of the Osvaldo Cruz Foundation (BA), protocol no. 84/2006. All the pregnant women were informed of the set of infectious agents being tested and authorized the collection of a greater quantity of blood than usual. Positive tests for women using the filter paper test were confirmed by the presence of antibodies specific to the infectious agents studied and the SMS of Lauro de Freitas was informed through the Active Search System, in order to locate the woman and provide her with the appropriate therapeutic and preventative intervention.
ResultsSamples of serum and DBS were obtained from 692 pregnant women aged between 14 and 42 years, with an average (SD) and median age of 27.1 (8.43) and 26 years, respectively. The average (SD) and median gestational ages at the time of blood collection were 15.78 (7.23) and 15 weeks, respectively. Serology was collected during the 1st (45%), 2nd (46.56%) and 3rd (8.45%) trimester of pregnancy. The reference units of the Centre for Women's Health and the Citizen Space carried out 30.6% and 14.3% of the total tests, respectively.
None of the samples were reagent for HCV and HIV on DBS or on serum tests. Thirteen pregnant women (1.88%; 95% CI: 0.60–2.71) presented with IgM serology reagent for T. gondii. Five pregnant women (0.72%; 95% CI: 0.15–1.61) tested positive for T. pallidum, two for HBV (0.29%; 95% CI: 0.05–0.95), and one for HTLV (0.14%; 95% CI: 0.01–0.71).
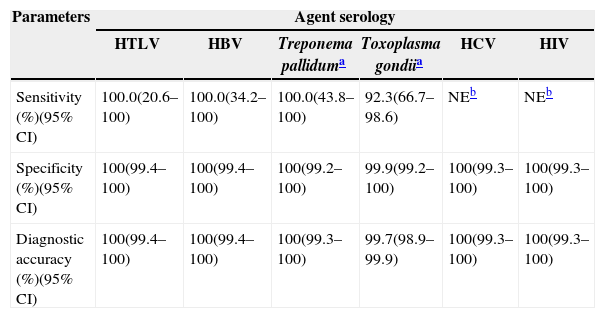
The sensitivity and specificity of DBS serology compared to serum ELISA are described in Table 1. Accuracy between the two serological procedures (DBS and serum) was 100% (95% CI: 99.25–100.0) for syphilis and 100% (95% CI: 99.45–100.0%) for HTLV (with a sensitivity of 100.0%; 95% CI: 20.6–100.0 and a specificity of 100%; 95% CI: 99.4–100.0).
Comparative accuracy between dried blood spots on filter paper and venous blood samples collected in tubes for laboratory use to validate a collection and serological screening method for HIV, HTLV, HBV, HCV, Treponema pallidum and Toxoplasma gondii in pregnant women, Lauro de Freitas, Bahia, 2009–2010.
| Parameters | Agent serology | |||||
|---|---|---|---|---|---|---|
| HTLV | HBV | Treponema palliduma | Toxoplasma gondiia | HCV | HIV | |
| Sensitivity (%)(95% CI) | 100.0(20.6–100) | 100.0(34.2–100) | 100.0(43.8–100) | 92.3(66.7–98.6) | NEb | NEb |
| Specificity (%)(95% CI) | 100(99.4–100) | 100(99.4–100) | 100(99.2–100) | 99.9(99.2–100) | 100(99.3–100) | 100(99.3–100) |
| Diagnostic accuracy (%)(95% CI) | 100(99.4–100) | 100(99.4–100) | 100(99.3–100) | 99.7(98.9–99.9) | 100(99.3–100) | 100(99.3–100) |
Average time (SD) between DBS collection and sample registration at APAE, Salvador was 4.93 (3.82) days and average time (SD) between processing the DBS material and an active search for the pregnant woman was 3.44 (4.27) days.
DiscussionThe most prevalent positive serological markers of the infections assessed in the studied pregnant women were for toxoplasmosis and syphilis. No sample was reagent for either HIV or HCV. DBS serology demonstrated a good level of accuracy compared to serum serology, and offered a probable reduction in elapsed time between testing and the municipal health system becoming aware of the result.
The average age of the studied pregnant women (27.1 years) was similar with the average age of the 2,956 pregnant women in the municipality of Lauro de Freitas in 2010 (26.6 yearsb). During the study period, 1183 prenatal consultations were recorded,c which represented a serological testing coverage of 58.5% of consultations. Given that there was more than one consultation per pregnant woman, and that the pregnant women in the second and third trimester, 46.6% and 8.45% respectively, did not undertake another DBS serological test during the study period, we obtained good coverage for those women who sought prenatal laboratorial assistance. These findings, associated with the fact that the tests were conducted in all public health units in the municipality that provide a laboratory collection service, suggest the appropriate representativeness of the study.
Most pregnant women had their serology tests in the second trimester of pregnancy, similar to what Lima and collaborators observed in Salvador, where 65% of the pregnant women only underwent their first serology tests during this period of pregnancy.5 These authors also reported the longer time taken in HIV and syphilis laboratory screening for low-income pregnant women.5 Although we did not assess income in this study, access to laboratory tests has become more difficult for low-income pregnant women, which reinforces the need to provide diagnostic methods during prenatal consultations that take place in UBS and Family Health Units (Unidades de Saúde Familiar: USFs).
The main problem related to late collection of serological tests in prenatal care (PNC) refers to delays in the initiation of prophylactic measures for vertical transmission of HIV and the immediate treatment of syphilis.3 According to the Ministry of Health, antiretroviral therapy should be initiated from the 14th week of gestation on.3 Data related to pregnant women in Salvador confirm the late initiation of PNC and the difficulty of conducting serological tests for HIV and VDRL during pregnancy.5
This study found a specific syphilis prevalence of 0.72% (95% CI: 0.15–1.61). A previous study of 1024 pregnant women, conducted in Salvador at the beginning of the 1990s, found a prevalence of 3.91% (95% CI: 2.72–5.09).26 In 2007 in the state of Sergipe, North East Brazil, a prevalence of 0.9% for syphilis was described in 9051 pregnant women.20 Over the last five years in Brazil, similar results have been observed in Mato Grosso,21 in the Central West region, and Botucatu (SP state),27 South East region. Greater prevalence has been observed in Vitoria (ES), South East,28 and Londrina (PR) South,29 at 3.6% and 1.6% respectively. Although the prevalence of syphilis among pregnant women varies across different regions of Brazil and between studies, the presence of this infection during pregnancy continues to be an important public health problem for the country6 and is associated with the maintenance of a significant incidence of congenital syphilis.
The HTLV-1 prevalence observed in the pregnant women we assessed was less than the 2.0% described in general female population of Salvador city in 1998,30 and in other studies among pregnant women in Salvador, where the prevalence has ranged from 0.84 to 0.98%,26,31 but similar to that observed in the Central West18,19,21,32 and South East27 of Brazil. However, more recently, the prevalence of the HTLV-1 infection in blood donors in Salvador fell from 1.35% in 19937 to 0.48% in 2005,33 a fact also found in the rest of Brazil.8
The reduction in syphilis and HTLV-1 prevalence observed in the pregnant women in the present study, as well as in more recent studies, as opposed to previous studies conducted in Salvador, may be due to the effect of HIV prevention campaigns and the enlarged support network for the diagnosis and prevention of STDs.33 These activities have had repercussions on primary prevention measures and on enhanced diagnostic knowledge, which implies a reduction in the transmission of these agents.
On the other hand, since there is a greater geographical proximity between Salvador and Lauro de Freitas, the prevalence differences between the two cities were unexpected. This finding may be partially explained by the different assessment periods, since our study is more recent, and therefore able to capture a prevalence reduction among pregnant women as already observed in blood donors7,8,26,33 and women aged 16–30 years old, which is the age of most of the pregnant women studied, and also given that HTLV-1 infection increases with age.30 The per capita income difference found between the municipalities of Lauro de Freitas and Salvador (R$17,026.64/US$ 9922.28 versus R$10,948.50/US$6380.24d) may also justify these differences, since HTLV-1 has been associated with lower income and less schooling.30
The other infectious agent found among these pregnant women was HBV, at a prevalence of 0.29% (95% CI: 0.05–0.95). These values were lower than those found in other Brazilian studies, including in the city of Salvador21,28,29,34,35 and international surveys in developing and low-income countries.36–38 Despite this, the prevalence of HBsAg was higher among the women studied than that of HIV and HCV, for which we did not find any seropositive woman, a finding repeated in most other federal units.21,30 However, HIV infection among pregnant Brazilian women was described in another study (seroprevalence 0.2%).39
The prevalence of anti-Toxoplasma IgM, suggestive of an acute infection, was the most frequent among these pregnant women, reaching 1.88% (95% CI: 0.6–2.71). These findings are similar to those from other regions, which range from 0.4 to 2.1%.20,21,27,40 Although this result indicates a possible acute T. gondii infection, its prevalence was lower than that observed in Salvador in the 1990s,26 which may reflect a reduction in acute infection during pregnancy as a result of the population's enhanced knowledge about exposure to infectious agents.
This study also assessed the possibility of the use of DBS as a tool for carrying out tests to detect antibodies against HIV, HTLV, HVB, HVC, T. pallidum and T. gondii through comparison with the same immune-trial conducted using serum. In particular, this study used the cross-sectional method, which is preferable to case–control studies, since the latter are subject to measurement bias of the predictor variable.41 By utilizing a cross-sectional method based on a pre-existing sample collection procedure for PNC serological screening in units that routinely carry out such tests, the study reproduces the clinical spectrum of disease incidence with respect to the capacity of the health system's structural network to detect such an event.
The diagnostic accuracy observed in this study was similar to that described in other studies conducted in Brazil21–23 and other countries,12,13,42 and corroborates the use of DBS as a viable and low cost tool with greater operational capacity and lower risk of contamination in transport and storage.12,14 The use of easier to acquire equipment – sterile lancets and filter paper – compared to those used for serological standard procedures – syringes, tubes, centrifuges, refrigerators and freezers – is one advantage for tests conducted on filter paper.12–14
The use of this method of transporting blood in regions at a distance from large centres and by UBS teams, such as those from the PSF, may increase access to serological tests, through a reduction in the turnaround time of results and the possibility of early intervention in the prevention of vertical transmission. In fact, the average time elapsed between blood collection on filter paper and result availability at the Department of Health did not surpass 10 days. We did not find studies that assessed the time elapsed between the serological test stages, although Vieira and collaborators have described the availability of tests as a relevant factor for pregnant woman.10 Despite this gap, several studies have demonstrated an association between lower income, less schooling, indicators of less accessibility to laboratory tests, and the lowest percentage for adequate PNC levels.1,2,4,5,9,10
In fact, it is reasonable to suppose that the process is more expeditious when one uses the DBS methodology integrated into the system of dispatch, processing of and active search for cases that fall within the UBS, USF, Laboratory Reference and SDTs/AIDS Care Centres. Additionally, a study conducted in Bahia to evaluate the heel prick test, demonstrated a median time of 35 days between birth and initiation of treatment for children with classical phenylketonuria.43
This study has certain limitations, such as the lack of evaluation of the final result of the obtained diagnosis (confirmation of treatment for the pregnant woman and serological and clinical screening for the newborn). Future investigations must be carried out that consider the role of the SUS structural network in prenatal, birth and puerperium care, since works with this focus of analysis have found discrepant results.1,10,44
Another limitation refers to the number of pregnant women studied, since the obtained sample size was inadequate for the identification of prevalence below 0.2%, which may explain the non-identification of pregnant women with HIV, since previous studies in Salvador have found a prevalence of around 0.1% for this agent.26 Despite this, coverage of practically all the serological tests conducted in the municipality during the study period ensured the good representativeness of the women studied and the accuracy of the data.
We may therefore say that the infectious agents that cause congenital infections continue to be relevant in the Metropolitan Region of Salvador, despite the decline observed in recent years. The use of DBS represents a secure and viable alternative to increase pregnant women's access to serological tests in the prenatal period, strengthening the role played by an association of this method with an active results information system and an active search for cases in order to reduce diagnosis the time elapsed and prevent vertical transmission.
FundingFundação de Amparo à Pesquisa do Estado da Bahia (FAPESB).
Conflicts of interestThe authors declare no conflicts of interest.
Data obtained from: http://tabnet.datasus.gov.br/CGI/tabcgi.exe?idb2010/f16def (in Portuguese) [accessed 07.06.12].
Data obtained from the DATASUS site: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sinasc/cnv/pnvba.def (in Portuguese) [accessed 20.02.12].
Data obtained from the DATASUS site: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sia/cnv/qaba.def (in Portuguese) [accessed 08.06.12].
Data obtained from the website of the Brazilian Institute of Geography and Statistics (Instituto Brasileiro de Geografia e Estatística: IBGE) http://www.ibge.gov.br/cidadesat/topwindow.htm?1 (in Portuguese) and http://www.ibge.gov.br/cidadesat/topwindow.htm?2 (in Portuguese) [accessed 20.02.12 and 07.06.12].
- Home
- All contents
- Publish your article
- About the journal
- Metrics