There is a paucity of data on the occurrence of congenital toxoplasmosis in children born to mothers dually infected with HIV and Toxoplasma gondii.
ObjectiveTo evaluate aspects of the mother–infant pairs associated with vertical transmission of toxoplasmosis in women co-infected with HIV in a referral center for perinatally acquired infections in Belo Horizonte, Brazil.
MethodsDescriptive study of HIV vertically exposed children, with congenital toxoplasmosis, followed at a referral center (cohort/Belo Horizonte). Prenatal and post-natal variables for the mother–infant pairs were evaluated. A literature review with no filtering for time and language was performed to identify reports of congenital toxoplasmosis in HIV vertically exposed children.
ResultsAmong 2007 HIV vertically exposed children evaluated in the period from 1998 to 2011, 10 cases of congenital toxoplasmosis were identified (incidence: 0.5%, 95% confidence interval: 0.24–0.91). In searching the literature 22 additional cases in 17 reports were found. Combining the findings of our cohort with other reported cases, 50% (16/32) of congenital toxoplasmosis in HIV vertically exposed children were from Brazil. The cases of congenital toxoplasmosis in HIV vertically exposed children identified in Brazil occurred mainly in the post-Highly Active Antiretroviral Therapy era (p=0.002) and presented a lower death rate (p=0.003) than those from other countries. In the cohort/Belo Horizonte, HIV infection was identified mainly during gestation; T. gondii vertical transmission was observed in pregnant women with CD4+>500cells/mm3 and latent toxoplasmosis. High rates of ocular lesions (87.5%) and central nervous system involvement (70%) were detected.
ConclusionsThe risk of vertical transmission of T. gondii in HIV-infected women is low and has been usually associated with maternal immunosuppression and elevated viral load. However, our findings of congenital toxoplasmosis in children born to HIV-infected mothers with latent toxoplasmosis and not immunosuppressed emphasize the need for careful follow-up in these cases.
Toxoplasma gondii infection has a worldwide occurrence, higher prevalence in tropical countries and is associated with weather conditions, cat population, and local life habits.1 Classical descriptions include three strains of the parasite: type 1 is usually associated with acute infection; type II is predominant in immunosuppressed individuals and in human congenital and eye infections; and type III is frequently isolated in animals.2 In Brazil, genetic diversity is greater than that in most other countries, and recombinant and atypical genotypes are predominant, usually associated with higher virulence.3–5
The prevalence of toxoplasmosis among pregnant women in Brazil range from 50% to 80%.6 In the metropolitan area of Belo Horizonte, state of Minas Gerais, where the current study was carried out, the seroprevalence is estimated in 60%.6 The prevalence of congenital infection is rare in the USA (approximately 0.01%) and higher in some Southern countries of Western Europe (0.01–0.1%).1,7 In Brazil, the prevalence is high throughout the country (0.05–0.15%), particularly in Minas Gerais state where it reaches 0.13%.8
In HIV-infected patients, most cases of toxoplasmosis result from the reactivation of a latent infection.9 In immunocompromised pregnant women with chronic toxoplasmosis, congenital transmission of the parasite probably occurs due to reactivation, with chronic and intermittent parasitemia.10–13 Diagnosis in these cases is a challenge, as they are usually associated with low IgG concentration and absence of IgM. For this reason, the protocol proposed by Lebech et al.,14 which is usually used to diagnose the parasite in the mother–infant pair, does not apply to immunosuppressed pregnant women.
The factors determining the various clinical manifestations of congenital toxoplasmosis are still poorly known. In fact, there may be a combination of factors, such as the host's genotype and immune status, the parasite tissue load, stage of placental development, and the parasite's own genetic composition.15–17 In immunosuppressed children co-infected with HIV and T. gondii, neurological damage is mainly a result of the toxoplasmosis congenital infection.18
Given the high prevalence of toxoplasmosis and the presence of more virulent strains of the parasite in Minas Gerais, the difficulty of diagnosing a new infection or reactivation in immunosuppressed pregnant women, and the paucity of studies on congenital toxoplasmosis among children of co-infected mothers, this paper reports a review of the literature and case studies of children with congenital toxoplasmosis that were born to HIV and T. gondii co-infected mothers. These children have been seen at a leading outpatient referral center in Belo Horizonte.
Materials and methodsThe study was divided into two parts: a descriptive study of congenital toxoplasmosis in a series of cases in a historical cohort of children vertically exposed to HIV and literature review on congenital toxoplasmosis in infants born to HIV-infected mothers.
Children included in the first part had been in follow-up at the Pediatric Immunology Center, Clinics Hospital, Federal University of Minas Gerais (UFMG), over the last 22 years (1998–2011). All mother–infant pairs are users of the public Brazilian unified health system (SUS) in the Metropolitan Region of Belo Horizonte and other municipalities in the State of Minas Gerais (Southeastern Brazil). The study included all children enrolled in the service that were born to HIV and T. gondii co-infected mothers, regardless of the infection stage. The criteria used to diagnose HIV infection and toxoplasmosis in the mothers are described below.
- 1.
HIV infection – positive for anti-HIV-1 and anti-HIV-2 in a screening test and in at least one confirmatory test. This included recommendations by the Centers for Disease Control and Prevention (CDC) and by the Brazilian Ministry of Health.19
- 2.
T. gondii infection – positive anti-T. gondii IgG whether or not associated with positive anti-T. gondii using enzyme linked immunosorbent assay and indirect immunofluorescence as detection methods. Determining the most likely moment of maternal infection involved: (a) acute infection – pregnant women with seroconversion: maternal IgG- and IgM-specific serologic tests with negative first results and positive second results; (b) undetermined, but probably recent, acute infection in the mothers: positive results for IgG- and IgM-specific serologic tests associated with low IgG avidity; and (c) chronic infection: mothers’ seropositive sample before conception or first serologic test carried out during pregnancy with positive IgG and negative IgM results or positive IgG and IgM results, both associated with high-avidity IgG antibodies.14
The diagnosis of congenital toxoplasmosis and HIV infection in children was performed according to the criteria described below.
- 1.
HIV infection: according to recommendations by the Centers for Disease Control and Prevention (CDC) and by the Brazilian Ministry of Health.19
- 2.
Congenital infection with T. gondii: according to criteria developed by Lebech et al.14 Present IgM and/or IgA anti-T. gondii in the first six months of life, persistently positive IgG anti-T. gondii at age of 12 months, or positive IgG associated with positive signs/symptoms and maternal seroconversion during pregnancy. Infection was ruled out in children with negative results for IgG tests in the first year of life, in the absence of anti-parasite treatment, or negative IgG after 1–3 months upon interrupting treatment in the first year of life.14
The study included all children born to HIV and T. gondii co-infected mothers who fulfilled the criteria for the diagnosis of congenital toxoplasmosis, regardless of their HIV status.
Women were considered immunosuppressed if CD4+ T lymphocyte were <350cells/mm3, and with severe immunosuppression if CD4+<200cells/mm3.19
In addition to the case studies, an extensive literature review was carried out on Pubmed, Scielo, and Cochrane Library databases. It included materials published until January 2012 and was not filtered for language and time period. The following descriptors were used: pregnancy AND toxoplasmosis AND HIV; congenital toxoplasmosis AND HIV; toxoplasmosis AND HIV.
Both the case studies and literature review targeted the variables related to the pregnant women (prenatal exams, symptomatology, immunosuppression level, and treatment) and to the children (birth conditions, diagnostic methods, symptomatology; anti-parasitic treatment, and case outcome). Information on the study participants was collected using structured questionnaires to capture information from the clinical charts. SPSS (Statistical Package for Social Sciences) version 17.0 was used to store and analyze the database. Description of the clinical and laboratory variables included absolute and relative frequency distribution, medians, and percentiles. For comparisons between proportions Fisher's exact test for small sample population was used. Medians of continuous variables were compared using Kruskal–Wallis’ test. Significance level was chosen at 5% (p<0.05).
The current study was approved by UFMG Ethics Committee (approval ETIC 260-03-EX 01/07).
ResultsA total of 2007 infants born to HIV-infected mothers were followed-up and systematically tested for toxoplasmosis in the first year of life. Out of this population, 10 children born to nine co-infected mothers were identified with congenital toxoplasmosis. Prenatal information of one of the women who gave birth in 1991 was not available in the database. Most women (7/8) were identified as HIV-infected during pregnancy. Timing of T. gondii infection could not be determined based on the analysis of maternal serologic tests. Two pregnant women (2/7) had clinical manifestations of toxoplasmosis, but were not provided specific treatment because the diagnosis was not confirmed upon delivery. One pregnant woman (1/7) was given sulfametoxazol and trimethoprim (SMX–TMP) as prophylaxis against pneumocystis during pregnancy, but its use was irregular. A great proportion of clinical manifestations were observed in the cohort/BH: eye and brain damage affected 87.5% and 70.0% of the children, respectively. As in the pregnant women, there was difficulty in diagnosing the disease due to lack of specific IgM and IgA during the first months of life (Table 1).
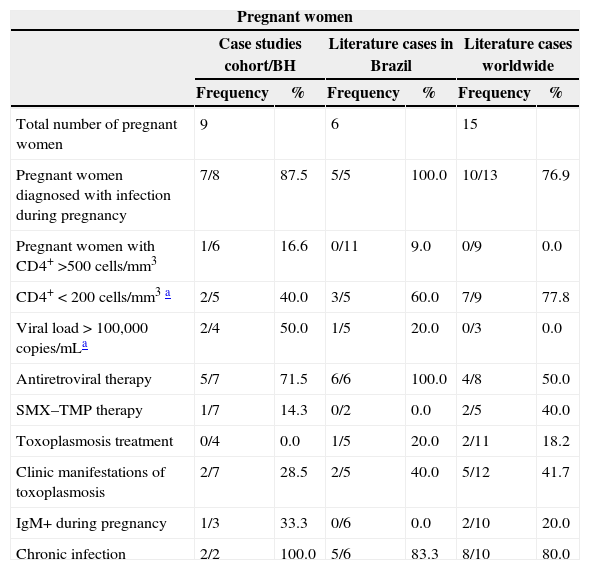
Frequency distribution of clinical and laboratory characteristics of children with congenital toxoplasmosis and their HIV/T. gondii co-infected mothers according to the children's place of origin (Metropolitan Area of Belo Horizonte, other regions in Brazil, and other countries).
| Pregnant women | ||||||
|---|---|---|---|---|---|---|
| Case studies cohort/BH | Literature cases in Brazil | Literature cases worldwide | ||||
| Frequency | % | Frequency | % | Frequency | % | |
| Total number of pregnant women | 9 | 6 | 15 | |||
| Pregnant women diagnosed with infection during pregnancy | 7/8 | 87.5 | 5/5 | 100.0 | 10/13 | 76.9 |
| Pregnant women with CD4+ >500cells/mm3 | 1/6 | 16.6 | 0/11 | 9.0 | 0/9 | 0.0 |
| CD4+<200cells/mm3a | 2/5 | 40.0 | 3/5 | 60.0 | 7/9 | 77.8 |
| Viral load>100,000copies/mLa | 2/4 | 50.0 | 1/5 | 20.0 | 0/3 | 0.0 |
| Antiretroviral therapy | 5/7 | 71.5 | 6/6 | 100.0 | 4/8 | 50.0 |
| SMX–TMP therapy | 1/7 | 14.3 | 0/2 | 0.0 | 2/5 | 40.0 |
| Toxoplasmosis treatment | 0/4 | 0.0 | 1/5 | 20.0 | 2/11 | 18.2 |
| Clinic manifestations of toxoplasmosis | 2/7 | 28.5 | 2/5 | 40.0 | 5/12 | 41.7 |
| IgM+ during pregnancy | 1/3 | 33.3 | 0/6 | 0.0 | 2/10 | 20.0 |
| Chronic infection | 2/2 | 100.0 | 5/6 | 83.3 | 8/10 | 80.0 |
| Children | ||||||
|---|---|---|---|---|---|---|
| Case studies cohort/BH | Literature cases in Brazil | Literature cases worldwide | ||||
| Frequency | % | Frequency | % | Frequency | % | |
| Total number of children | 10 | 6 | 16 | |||
| Pre-HAART | 1/10 | 10.0 | 0/6 | 0.0 | 11/16 | 68.75 |
| Term birth | 5/9 | 55.5 | 3/5 | 60.0 | 6/13 | 46.1 |
| HIV co-infected children | 5/9 | 55.5 | 3/5 | 60.0 | 10/12 | 83.3 |
| Chorioretinitis | 7/8 | 87.5 | 4/5 | 80.0 | 5/8 | 62.5 |
| Brain damage | 7/10 | 70.00 | 2/4 | 50.0 | 8/10 | 80.0 |
| Systemic symptoms of toxoplasmosis | 4/4 | 100.0 | 2/5 | 40.0 | 11/12 | 91.7 |
| Test for toxoplasmosis in the first quarter | 8/10 | 80.0 | 6/6 | 100.0 | 14/16 | 87.5 |
| Anti-T. gondii IgM/IgA or PCR+at birth | 4/10 | 40.0 | 6/6 | 100.0 | 7/12 | 58.3 |
| Result through autopsy | 0/2 | 0.0 | 1/1 | 100.0 | 11/12 | 91.7 |
| Treatmentb | 8/10 | 80.0 | 5/5 | 100.0 | 7/10 | 70.0 |
| Mortalityc | 2/10 | 20.0 | 1/4 | 25.0 | 12/15 | 80.0 |
The highest viral load value and the lowest CD4+ value were used referring necessarily to a given moment in the pregnancy. The level of 20% of CD4+ lymphocytes corresponded to an absolute count at 350cells/mm3.
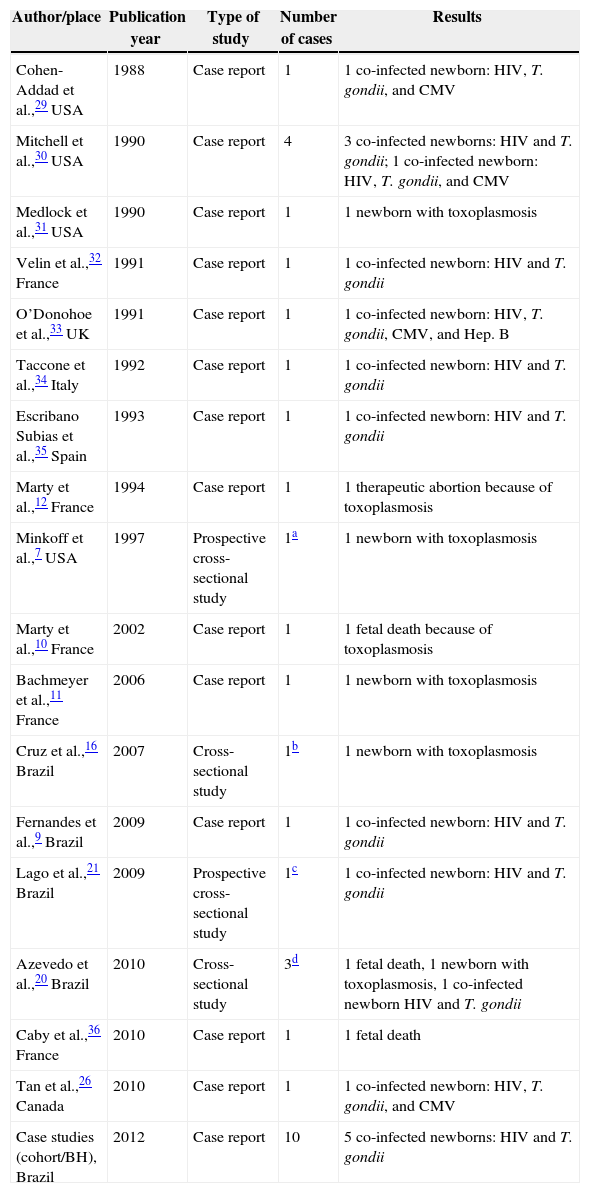
In total, the bibliographic review retrieved 17 articles with 22 cases of congenital toxoplasmosis in children of co-infected mothers. Four articles reported cross-sectional studies. Congenital toxoplasmosis was rare and associated with maternal immunosuppression during pregnancy.7 Only four articles were written by Brazilian researchers reporting six cases altogether9,16,20,21 (Table 2).
Distribution of studies on children with congenital toxoplasmosis born to HIV and T. gondii co-infected mothers, according to year and place.
| Author/place | Publication year | Type of study | Number of cases | Results |
|---|---|---|---|---|
| Cohen-Addad et al.,29 USA | 1988 | Case report | 1 | 1 co-infected newborn: HIV, T. gondii, and CMV |
| Mitchell et al.,30 USA | 1990 | Case report | 4 | 3 co-infected newborns: HIV and T. gondii; 1 co-infected newborn: HIV, T. gondii, and CMV |
| Medlock et al.,31 USA | 1990 | Case report | 1 | 1 newborn with toxoplasmosis |
| Velin et al.,32 France | 1991 | Case report | 1 | 1 co-infected newborn: HIV and T. gondii |
| O’Donohoe et al.,33 UK | 1991 | Case report | 1 | 1 co-infected newborn: HIV, T. gondii, CMV, and Hep. B |
| Taccone et al.,34 Italy | 1992 | Case report | 1 | 1 co-infected newborn: HIV and T. gondii |
| Escribano Subias et al.,35 Spain | 1993 | Case report | 1 | 1 co-infected newborn: HIV and T. gondii |
| Marty et al.,12 France | 1994 | Case report | 1 | 1 therapeutic abortion because of toxoplasmosis |
| Minkoff et al.,7 USA | 1997 | Prospective cross-sectional study | 1a | 1 newborn with toxoplasmosis |
| Marty et al.,10 France | 2002 | Case report | 1 | 1 fetal death because of toxoplasmosis |
| Bachmeyer et al.,11 France | 2006 | Case report | 1 | 1 newborn with toxoplasmosis |
| Cruz et al.,16 Brazil | 2007 | Cross-sectional study | 1b | 1 newborn with toxoplasmosis |
| Fernandes et al.,9 Brazil | 2009 | Case report | 1 | 1 co-infected newborn: HIV and T. gondii |
| Lago et al.,21 Brazil | 2009 | Prospective cross-sectional study | 1c | 1 co-infected newborn: HIV and T. gondii |
| Azevedo et al.,20 Brazil | 2010 | Cross-sectional study | 3d | 1 fetal death, 1 newborn with toxoplasmosis, 1 co-infected newborn HIV and T. gondii |
| Caby et al.,36 France | 2010 | Case report | 1 | 1 fetal death |
| Tan et al.,26 Canada | 2010 | Case report | 1 | 1 co-infected newborn: HIV, T. gondii, and CMV |
| Case studies (cohort/BH), Brazil | 2012 | Case report | 10 | 5 co-infected newborns: HIV and T. gondii |
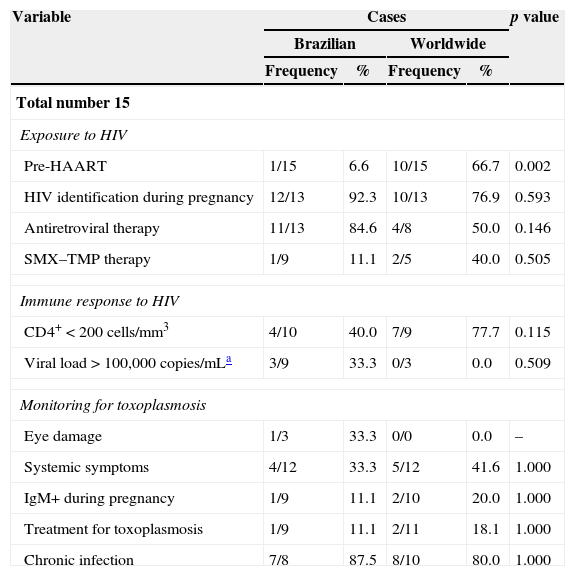
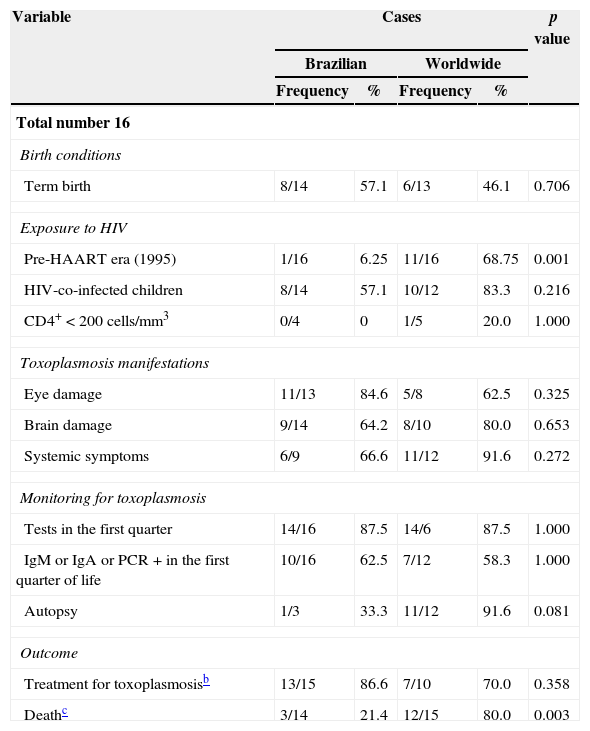
Comparison of pregnant women and children according to place of origin (Brazil vs. other countries) points to similar characteristics. However, only one Brazilian pregnant woman was diagnosed in the pre-HAART (Highly Active Antiretroviral Therapy) era, which contrasts with the pregnant women from other countries (p=0.002). The group of pregnant women from other countries included twice as many women with CD4+<200cells/mm3 than the group of Brazilian women, but this difference proved not to be significant. Differences between child mortality rates were found significant between places of origin (p=0.003): 80% of the deaths among children from other countries, out of which 92% had toxoplasmosis diagnosed post-mortem (Tables 3 and 4).
Frequency distribution of pregnancy characteristics of HIV/T. gondii co-infected mothers of children with congenital toxoplasmosis, according to the children's place of origin (Metropolitan Area of Belo Horizonte, other regions in Brazil, and other countries).
| Variable | Cases | p value | |||
|---|---|---|---|---|---|
| Brazilian | Worldwide | ||||
| Frequency | % | Frequency | % | ||
| Total number 15 | |||||
| Exposure to HIV | |||||
| Pre-HAART | 1/15 | 6.6 | 10/15 | 66.7 | 0.002 |
| HIV identification during pregnancy | 12/13 | 92.3 | 10/13 | 76.9 | 0.593 |
| Antiretroviral therapy | 11/13 | 84.6 | 4/8 | 50.0 | 0.146 |
| SMX–TMP therapy | 1/9 | 11.1 | 2/5 | 40.0 | 0.505 |
| Immune response to HIV | |||||
| CD4+<200cells/mm3 | 4/10 | 40.0 | 7/9 | 77.7 | 0.115 |
| Viral load>100,000copies/mLa | 3/9 | 33.3 | 0/3 | 0.0 | 0.509 |
| Monitoring for toxoplasmosis | |||||
| Eye damage | 1/3 | 33.3 | 0/0 | 0.0 | – |
| Systemic symptoms | 4/12 | 33.3 | 5/12 | 41.6 | 1.000 |
| IgM+ during pregnancy | 1/9 | 11.1 | 2/10 | 20.0 | 1.000 |
| Treatment for toxoplasmosis | 1/9 | 11.1 | 2/11 | 18.1 | 1.000 |
| Chronic infection | 7/8 | 87.5 | 8/10 | 80.0 | 1.000 |
Comparison between Brazilian and international cases according to children's characteristics.
| Variable | Cases | p value | |||
|---|---|---|---|---|---|
| Brazilian | Worldwide | ||||
| Frequency | % | Frequency | % | ||
| Total number 16 | |||||
| Birth conditions | |||||
| Term birth | 8/14 | 57.1 | 6/13 | 46.1 | 0.706 |
| Exposure to HIV | |||||
| Pre-HAART era (1995) | 1/16 | 6.25 | 11/16 | 68.75 | 0.001 |
| HIV-co-infected children | 8/14 | 57.1 | 10/12 | 83.3 | 0.216 |
| CD4+<200cells/mm3 | 0/4 | 0 | 1/5 | 20.0 | 1.000 |
| Toxoplasmosis manifestations | |||||
| Eye damage | 11/13 | 84.6 | 5/8 | 62.5 | 0.325 |
| Brain damage | 9/14 | 64.2 | 8/10 | 80.0 | 0.653 |
| Systemic symptoms | 6/9 | 66.6 | 11/12 | 91.6 | 0.272 |
| Monitoring for toxoplasmosis | |||||
| Tests in the first quarter | 14/16 | 87.5 | 14/6 | 87.5 | 1.000 |
| IgM or IgA or PCR+in the first quarter of life | 10/16 | 62.5 | 7/12 | 58.3 | 1.000 |
| Autopsy | 1/3 | 33.3 | 11/12 | 91.6 | 0.081 |
| Outcome | |||||
| Treatment for toxoplasmosisb | 13/15 | 86.6 | 7/10 | 70.0 | 0.358 |
| Deathc | 3/14 | 21.4 | 12/15 | 80.0 | 0.003 |
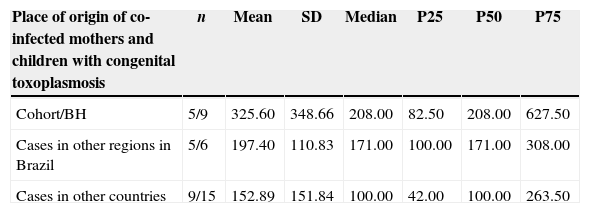
Several cases of vertical toxoplasmosis transmission were observed among the immunosuppressed pregnant women, but this also took place among women with more than 500 CD4+ cells (Table 4). In the HIV-infected cohort from Belo Horizonte, the mean absolute CD4+ values were higher than that in other settings. This was probably due to the wider variation on CD4+ values (36–917 CD4+cells/mm3) (Table 5).
Comparison of the lowest absolute values of T CD4+ lymphocytes (cells/mm3) during the pregnancy of HIV and T. gondii co-infected women and their children with congenital toxoplasmosis, according to place of origin.
| Place of origin of co-infected mothers and children with congenital toxoplasmosis | n | Mean | SD | Median | P25 | P50 | P75 |
|---|---|---|---|---|---|---|---|
| Cohort/BH | 5/9 | 325.60 | 348.66 | 208.00 | 82.50 | 208.00 | 627.50 |
| Cases in other regions in Brazil | 5/6 | 197.40 | 110.83 | 171.00 | 100.00 | 171.00 | 308.00 |
| Cases in other countries | 9/15 | 152.89 | 151.84 | 100.00 | 42.00 | 100.00 | 263.50 |
Kruskal–Wallis test – p=0.437.
The Belo Horizonte cohort (10 children born to 9 women) represents the highest number of children with congenital toxoplasmosis born to HIV and T. gondii co-infected mothers that have been reported by a single center. Considering the total cases of children with congenital toxoplasmosis born to co-infected mothers reported in the literature, half of them were from Brazil, with the cohort/BH representing approximately one-third of the total.
Occurrence of congenital toxoplasmosis in children born to HIV and T. gondii co-infected mothers is rare, as shown in the cohort/BH and the few cases reported worldwide. The cohort/BH, however, points out the need for investigating T. gondii infection systematically to diagnosis congenital toxoplasmosis among newborns that have been exposed to HIV. It also shows that, even in the post-HAART area, children are still at risk of acquiring congenital toxoplasmosis, and it is pertinent to discuss the best approach to co-infected pregnant women and their children. In addition, it contributes to reinforcing the association between vertical T. gondii transmission and chronic infection in immunosuppressed women. Additionally, this two-decade long observational study indicates that pregnant women transmitted toxoplasmosis to their children even when they did not have severe immunosuppression or kept absolute CD4+ values within normal limits.
A recent study has estimated the prevalence of congenital toxoplasmosis at 0.13% in the general population in the state of Minas Gerais, one infected child in every 770 live births.8 Considering this estimate and the number of HIV-exposed children followed up in the cohort/BH (n=2007), the expected estimate before this study was two or three children with congenital toxoplasmosis. Therefore, 10 infected children, as identified in the present study, represent a prevalence that is at least three times higher than expected in newborns of HIV-uninfected pregnant women. Since the Brazilian environment is favorable to T. gondii infection, HIV infection still occurs among mothers in reproductive age, and as congenital toxoplasmosis manifests somewhat later in the newborns’ lives, these cases might have been underestimated in our country.
In the present study we were not able to ascertain the risk of toxoplasmosis transmission among co-infected pregnant women. The European Collaborative Study has estimated it to be lower than 4.2%.22 This study, carried out in the pre-HAART era 1985–1999, included 1058 children born to 981 European HIV-infected women estimated maternal prevalence of toxoplasmosis of 42.6%, based on specific IgG detection in infants tested before their third month of age. They did not differentiate acute from chronic T. gondii infection and reported that only 18% of the mothers had CD4+ lymphocyte count below 200cells/mm3 at delivery, and 137 children had documented HIV perinatal infection. Other international studies predicted this risk, and the European AIDS surveillance system has reported toxoplasmosis occurrence in only 25 of the 1762 (1.4%) cases of vertically HIV-infected children reported until late 1994. Nevertheless, it is unknown whether such cases were due to congenital or acquired infection. Unfortunately, these surveillance systems do not include seroreverting children born to HIV positive mothers, which prevent the assessment of the risk of toxoplasmosis transmission among these co-infected pregnant women.22 Minkoff et al.7 indicated a rate of 3.7% of vertical Toxoplasma transmission among HIV-infected women, but transmission could take place in up to 33% of the cases if the women are severely immunocompromised. However, the low prevalence of toxoplasmosis in the sample of pregnant women (20%) and the small sample size limited the study impact. Although there are three prospective studies from Brazil, not enough data are available to determine the risk of transmission.16,20,21
A prospective study was carried out in the state of Rio Grande do Sul, located in southern Brazil, from 2002 through 2005, following up 121 HIV and T. gondii co-infected pregnant women, to investigate the serologic profile of co-infected pregnant women and the risk of transmission of congenital toxoplasmosis. One child was identified with congenital toxoplasmosis, who, according to the authors, was born to a mother that had acquired the infection during pregnancy and did not present either high IgG concentration levels or IgM antibodies. The study also compared the IgG anti-T. gondii concentration level among the co-infected pregnant women and 1624 HIV negative pregnant women. The authors concluded that high T. gondii-specific IgG concentration levels were much more frequent among HIV infected pregnant women, but without statistical significance; high concentration, therefore, was not associated with an increased risk of mother–fetus transmission of toxoplasmosis.21 Another study followed up 767 HIV-exposed children in the city of Rio de Janeiro, Brazil, from 1996 through 2007. Only one child had congenital toxoplasmosis, and 74% of the mothers had serological evidences of IgG anti-Toxoplasma during pregnancy.16 A third study, also in Rio de Janeiro, describes three children with congenital toxoplasmosis out of the 113 HIV-exposed children. In the sample, 70% of the pregnant women had serological (IgG positive) evidence of T. gondii infection during pregnancy.20 A fourth study, also carried out in Rio de Janeiro (1996–2001), consisted of a cohort of 227 HIV-infected pregnant women, and found a prevalence of chronic T. gondii infection among 71.4% of them.23,24 Five children of this cohort were infected with HIV, but no case of congenital toxoplasmosis was diagnosed. The women with CD4+<200cells/mm3 were provided with SMX–TMP three times a week during pregnancy.24 This prophylactic regimen was used for pneumocystis prevention, but may have contributed to preventing reactivation of latent toxoplasmosis, which may have accounted for a rare occurrence of vertical transmission.25
Altogether, these data call for caution in interpreting the estimate of 4% risk of vertical toxoplasmosis transmission in co-infected pregnant women that has been reported in the literature, as the population under scrutiny is heterogeneous. This risk can be higher among co-infected women with HIV and chronic T. gondii infection when they have severe immunosuppression. Another factor that may affect vertical transmission is the use of antimicrobials as a prophylactic measure against pneumocystis by immunosuppressed pregnant women.11,25
The finding of just one child born of the cohort/BH in the pre-HAART area may be explained by the developments at the specialized outpatient referral center over time: only recently has it introduced standards for reportable cases, follow-up protocols, and systematic performance of serologic tests among pregnant women at risk. These developments also explain the difference between national and international death rates. The systematic study of toxoplasmosis in newborns in Minas Gerais allowed for diagnosing the disease regardless of evident clinical damage.
Several challenges exist to diagnosing congenital transmission of the infection building on its clinical markers. Unfortunately, the Lebech et al.14 criteria used to diagnose toxoplasmosis in pregnant women and children do not include cases of immunosuppression. Clinical manifestations that might prompt suspicions of infection are usually absent or belated; 80–90% are asymptomatic at birth, regardless of the co-infection. Tan et al. have reported a case of a co-infected newborn that reputedly had negative results for tomography of the skull and fundoscopy, and could not be diagnosed with toxoplasmosis before the 50th day of life. This case underscores the difficulty of diagnosing congenital toxoplasmosis among HIV-co-infected individuals and the need for repeating diagnostic tests and even empiric therapy, because clinical and radiographic abnormalities may be identified belatedly and the results may be negative in the first serological tests.26 Explanations for this may be: intrauterine exposure to the SMX–TMP used as a prophylaxis by the mother; disappearance of anti-T. gondii IgM during fetal life and its non-detection at birth; and the possibility of several congenital co-infections that might share overlapping clinical features, such as CMV. Another explanation is that simultaneous HIV infection may contribute to failed IgG immunologic response to T. gondii.26
For laboratory diagnosis of toxoplasmosis, increased antibody concentrations can be found in the cases of reactivations in HIV infected individuals, including pregnant women with CD4+>200cells/mm3. In primo-infection, paradoxically, humoral response may be reduced against the recently introduced antigens.20 Reinfection by different strains may not lead to specific IgM seroconversion.11 Avidity of IgG antibodies, if carried out at the beginning of the pregnancy, may be useful in assessing the risk of vertical transmission, since they differentiate a recently acquired infection from an old infection.16 It is not prudent to diagnose fetal infection through parasite DNA detection in amniotic liquid because of the risk of infecting the fetus with HIV during the procedure on co-infected pregnant women.26 These results pose difficulties in dealing with suspected cases of toxoplasmosis and implementing treatment measures aimed at the newborn. Therefore, to increase sensitivity to confirm suspected cases among newborns, physicians should perform IgG, IgM, and IgA specific tests in the immediate postnatal period. Initial negative results should imply repeating the tests. Parasite DNA tests through PCR of the children's blood or cerebro-spinal fluid sample may contribute to an accurate diagnosis.
Several authors advise that all infants born to HIV seropositive mothers should be tested for congenital infections. Physicians should not miss the opportunity to treat children from their birth or even during their intrauterine life (in recommended cases).20,26 No consensus exists in relation to toxoplasmosis prophylaxis during the prenatal period. Some authors contend that anti-T. gondii IgG positive women with CD4+<100cells/mm3 should be given an antimicrobial prophylaxis during pregnancy to reduce the risk of transmitting the parasite to the fetus. They further argue that this procedure may also be appropriate for non-HIV-infected patients that are highly immunocompromised for other reasons.7 An article by Marty et al. suggests that chemoprophylaxis with SMX–TMP associated with antiretroviral treatment should be provided to HIV and toxoplasmosis seropositive pregnant women when they have CD4+<200cells/mm3 and high viral load.10,25 Other studies suggest that the risk of maternal–fetal transmission is not sufficiently high to justify routine anti-toxoplasmosis chemoprophylaxis in HIV-infected pregnant women, but they nevertheless emphasize the need for further research on the matter.22 As the risk of maternal–fetal transmission is not well known, particularly in countries like Brazil, which have high rates of T. gondii seroprevalence, the current obstetrical tendency of not prescribing anti-toxoplasmosis chemoprophylaxis to chronically infected women is understandable.20
It is recommended that specific treatment against Toxoplasma be initiated in HIV and toxoplasmosis co-infected children for one year, after which secondary prophylaxis should be provided. In Brazil, the recommendation is to hold back on secondary chemoprophylaxis until two consecutive assessments prove that CD4+ lymphocyte is higher than 15%.9
Although the small sample size is a limitation of this study, it is important to emphasize that this is a cohort of two decades with systematic investigation of toxoplasmosis in infants born to HIV infected mothers, which allowed identification of vertical transmission in HIV infected pregnant women with chronic toxoplasmosis. Incompleteness of study variables is a common bias encountered in retrospective studies. This limitation was minimized in the present study by the existence of standardized clinical forms used for the follow-up of both cohorts.
The authors of this study underscore the importance of diagnosis and treating toxoplasmosis in the prenatal period, along with HIV monitoring during the entire pregnancy. Immunodepression, even when mild, may contribute to the transmission of latent infections to the newborn, and this could be averted with appropriate treatment. Immunocompromised women should be tested for toxoplasma and cytomegalovirus infection in multiple occasions throughout their pregnancy. Successful cases have shown that adequate measures preserve the mothers’ health and avoid fetus infection in this scenario.23,27,28
ConclusionTo date, the general risk of maternal–fetal transmission of Toxoplasma infection is deemed to be low among HIV-infected women, even when their CD4+ lymphocyte counts are very low (<200cells/mL). However, the cohort/BH had a higher number of cases of congenital toxoplasmosis than expected among children born to co-infected mothers. Despite the discrepancy, these data should be interpreted with caution, due to the heterogeneity of the population under scrutiny, classification biases that hinder association between transmission and acute or chronic T. gondii infection, insufficient sample sizes to detect cases of the disease, and the paucity of prospective studies monitoring the mother/child duo.
This risk is also estimated to be low in countries with a high prevalence of toxoplasmosis, such as Brazil, and associated with the mothers’ severe immunodeficiency (CD4+<200cells/mm3) and high viral load. However, the occurrence of the disease among asymptomatic pregnant women with latent toxoplasmosis and CD4+ within normal limits underscores the need for careful monitoring of both mother and child in these cases. Further studies are needed to determine the effect of chemoprophylaxis on the transmission rates. The authors recommend that toxoplasmosis tests be carried out in all infants born to HIV-seropositive mothers.
Conflicts of interestThe authors declare no conflicts of interest.