Currently, hospital infection is a serious public health problem, and several factors may influence the occurrence of these infections, including the presence of insects, which are carriers of multidrug-resistant bacterial species. The aim of this study was to isolate staphylococci carried by insects in two public hospitals of Vitoria da Conquista, Bahia and to identify the resistance profile, pathogenicity and efficacy of disinfection of the premises. A total of 91 insects were collected in 21 strategic points of these hospitals, and 32 isolated strains of Staphylococcus aureus were isolated. Based on antibiogram and Minimum Inhibitory Concentration results, 95% of these strains were susceptible to oxacillin. These strains were also evaluated for the presence of resistance genes encoding resistance to oxacillin/methicillin by polymerase chain reaction, but the sample was negative for this gene. Pathogenicity tests were performed in vitro biofilm formation induced by glucose, where it was found that eight (27.58%) strains were classified as biofilm producers and 21 (72.4%) as stronger producers. In addition, we performed PCR for their virulence genes: Sea (enterotoxin A), SEB (B), Sec (C), PVL (Panton-Valentine Leukocidin), ClfA (clumping factor A) and Spa (protein A). Of these, Sea, Spa PVL were positive in 7 (21.8%), 2 (6.3%) and 1 (3.1%) samples, respectively. The analysis of cytokine induction in the inflammatory response of J774 macrophages by isolates from the two hospitals did not show statistical difference at the levels of IL-6, TNF-α, IL-1 and IL-10 production. In addition, we verified the antimicrobial activity of disinfecting agents on these strains, quaternary ammonium, 0.5% sodium hypochlorite, 1% sodium hypochlorite, 2% sodium hypochlorite, 2% glutaraldehyde, Lysoform®, 70% alcohol solution of chlorhexidine digluconate, 2% peracetic acid, and 100% vinegar. Resistance was seen in only for the following two disinfectants: 70% alcohol in 31 (96.8%) samples tested and vinegar in 30 (93.8%) samples. The study demonstrated the presence of resistant and pathogenic organisms conveyed by insects, thus suggesting improvement in efforts to control these vectors.
The Brazilian Ministry of Health1 defines Hospital Infection (HI) as the infection acquired after admission and manifested in patients during hospitalization or after hospital discharge, being possibly related to hospitalization or hospital procedures. HI is presented as an issue of great epidemiological significance due to significant human and economic losses.2
HI is also associated with environmental factors, where insects are present. These organisms are mechanical vectors of pathogens and can disperse multidrug-resistant strains, increasing the risk of severe community-acquired infections. Several factors may be associated with the presence of insects in a hospital environment, such as the physical structure of the hospital, including old and deteriorating buildings, proximity to nearby housing and food, a high flow of people and body fluids of patients.3
The main bacterial agent involved in nosocomial infections and transmitted by insects is Staphylococcus aureus. These microorganisms are Gram-positive cocci, catalase-positive, about 1.0mm in diameter, immobile, non-endospore-forming and usually non-encapsulated.4 These organisms are part of the human resident microbiota, commonly colonizing skin and mucosa of healthy people, being a versatile pathogen capable of causing a wide variety of human diseases.
The genus Staphylococcus is among the numerous pathogens that have undergone significant changes in antimicrobial susceptibility in recent decades.5 In addition to resistance, pathogenicity of these organisms is an extremely important feature to be understood. The ability of S. aureus to cause various infections and toxicity, results from the production of different extracellular and surface virulence factors with adhesive properties for a range of molecules (MSCRAMMs). The extracellular products include especially toxins with superantigenic properties, namely enterotoxins A-E, G-K, M-O and Q, exfoliative toxins A and B, toxic shock syndrome toxin-1 as well as, for example, Panton-Valentine Leukocidin.6 Another mechanism of resistance and pathogenicity is biofilm production. This mechanism occurs through the production of extracellular polysaccharide substances, causing bacterial cells to form clusters in multilayer biofilm, thus preventing the action of antibiotics and immune system.7
Over the last few decades, antimicrobial resistance has been well studied. However, bacterial resistance to sanitizing products is still poorly understood and studied. In clinical practice, antiseptics and disinfectants are used extensively in hospitals and other health environments for a variety of applications. Sanitizing and disinfectant agents are essential components in the practice of control and prevention of nosocomial infections. Some agents are more effective against Gram-positive than Gram-negative bacteria, necessitating an evaluation of their effectiveness. The aim of the present study was to: isolate S. aureus strains resistant to antibiotics carried by insects; characterize the genotypic profiles of staphylococci pathogenicity; and evaluate the efficacy of antiseptic agents and disinfectants used in two public hospitals of Vitória da Conquista, Bahia.
Materials and methodsSample collection and bacterial isolatesLive insects (ants, cockroaches, flies, wasps, gnats, moths and butterflies) were collected in two public hospitals of Vitória da Conquista. The sampling sites were randomly selected: meeting rooms, hallways, windows, emergency room, medical clinics, surgical clinics, reception areas and janitor storage areas. A total of 91 insects were collected in two public hospitals, being 31 in hospital one and 60 in hospital two. The insects were placed into tubes with 10mL of TSB, avoiding animal fragmentation for laboratory analysis. This procedure was performed to verify the microorganisms that were located on the surface of the insect. The samples were transported in 10mL of sterile modified culture medium BHI (Brain Heart Infusion) at 4°C. Broth cultures were plated onto mannitol salt agar. Cultures were incubated at 37°C for 48h. Suspect colonies, which revealed acidification of mannitol, were subjected to identification procedures. Colonies with coagulase-positive, Gram-positive cocci and catalase positive were selected as possible S. aureus and identified by PCR.
Susceptibility testingAntimicrobial susceptibility testing was performed by the broth microdilution method, following recommendations of the Clinical and Laboratory Standards Institute.8 Oxacillin and vancomycin were obtained from the respective manufacturers, and the plates were prepared and used on the same day as testing. The strain was subcultured in mannitol salt agar at 37°C overnight. On the day of experiment, bacterial suspension was prepared in 0.9% sodium chloride solution and the inoculum was adjusted by spectrophotometer. Susceptibility results were interpreted according to a CLSI document. The tests were read 24h after incubation at 35°C. Quality control was performed by testing S. aureus ATCC 29213 and ATCC 43300. All experiments were performed in triplicate with three independent repetitions.
Genotypic characterization to resistance and pathogenic genesMRSA strains were submitted to DNA extraction by the boiling method.9 The primers mecA1 and mecA2 were used in a PCR to detect the mecA gene.10 Moreover, all the isolates were submitted to PCR11 for detection of virulence factor genes: sea (Staphylococcal enterotoxins type A), seb (Staphylococcal enterotoxins type B), sec (Staphylococcal enterotoxins type C), PVL (Panton-Valentine Leukocidin), ClfA (clumping factor) and Spa (IgG-binding region and the X-region of protein A). Amplified products were separated by agarose gel electrophoresis (1% agarose containing 0.5mg ethidium bromide in 0.5× Tris–EDTA electrophoresis buffer) at 100V and photographed under UV illumination.
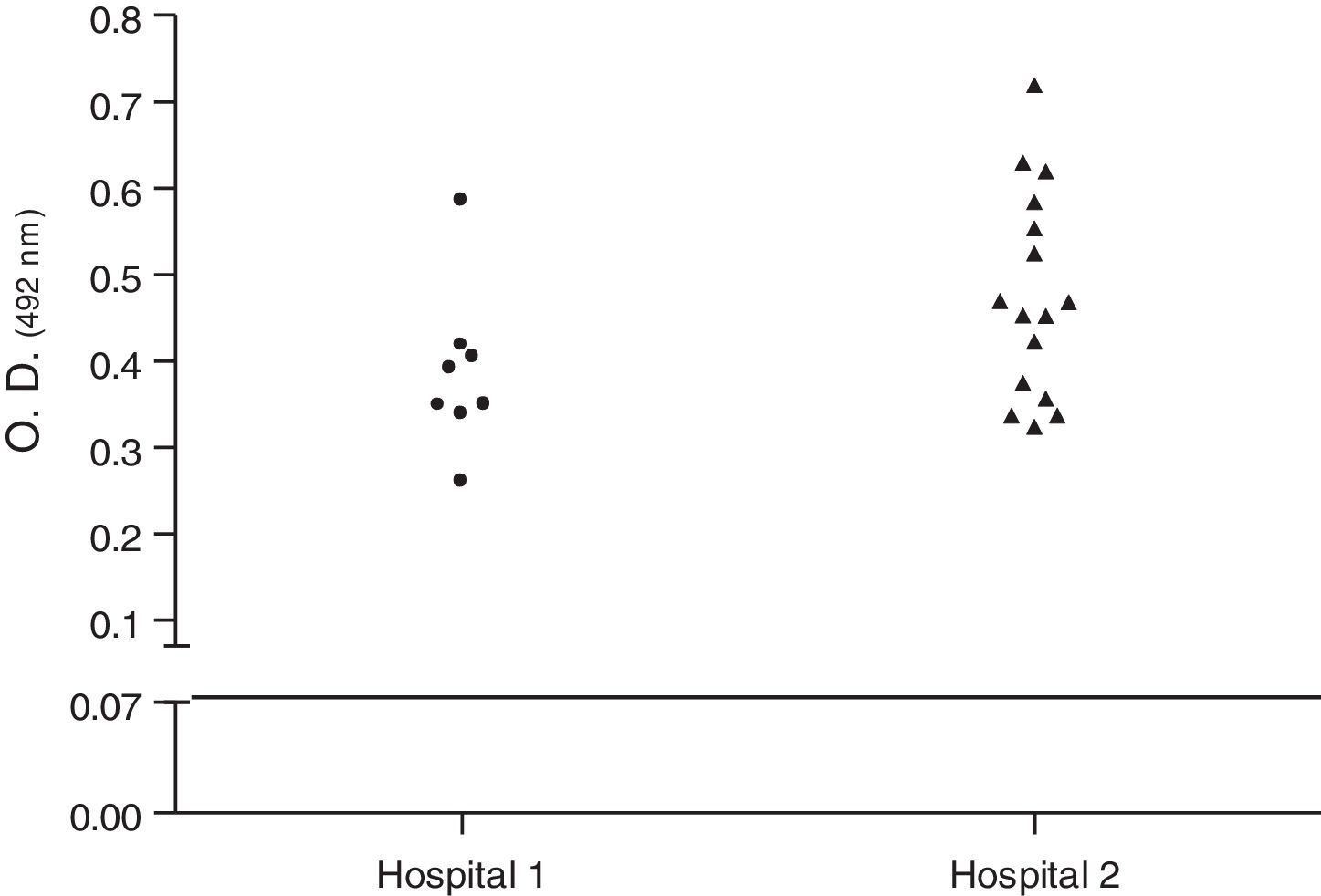
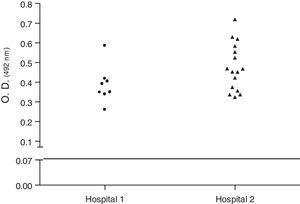
Biofilm productionBiofilm assays were performed in 96-well polystyrene microplates, using trypticase soy broth (TSB/Difco) with 1% (w/v) glucose (TSB-1% Glc).12 Briefly, cultures of staphylococi in 5mL were incubated in a shaker with 250rpm at 37°C for 18h. Cultures were diluted 1:100 in TSB-1% Glc and 200μL were inoculated into each well. The microplate was incubated at 37°C for 20h. Supernatants were removed from each well and biofilms were gently washed twice with PBS, then dried and fixed at 65°C for 1h. Finally, the plates were stained with crystal violet 1% used in Gram-stain and gently washed twice with PBS. The absorbance at 492nm was calculated in a spectrophotometer. The samples were compared with cultures of Streptococcus pyogenes ATCC75194. The S. aureus isolates were classified as non-biofilm producers, weak producers, moderate producers, producers, and strong producers. Because the production of biofilm depends on phase variation, tests were repeated four times. At least two independent experiments were carried out for each test. The cutoff point for the production was taken into account, the absorbance obtained by S. pyogenes (O.D.492 0.07). The mean value was used for the statistical calculation.
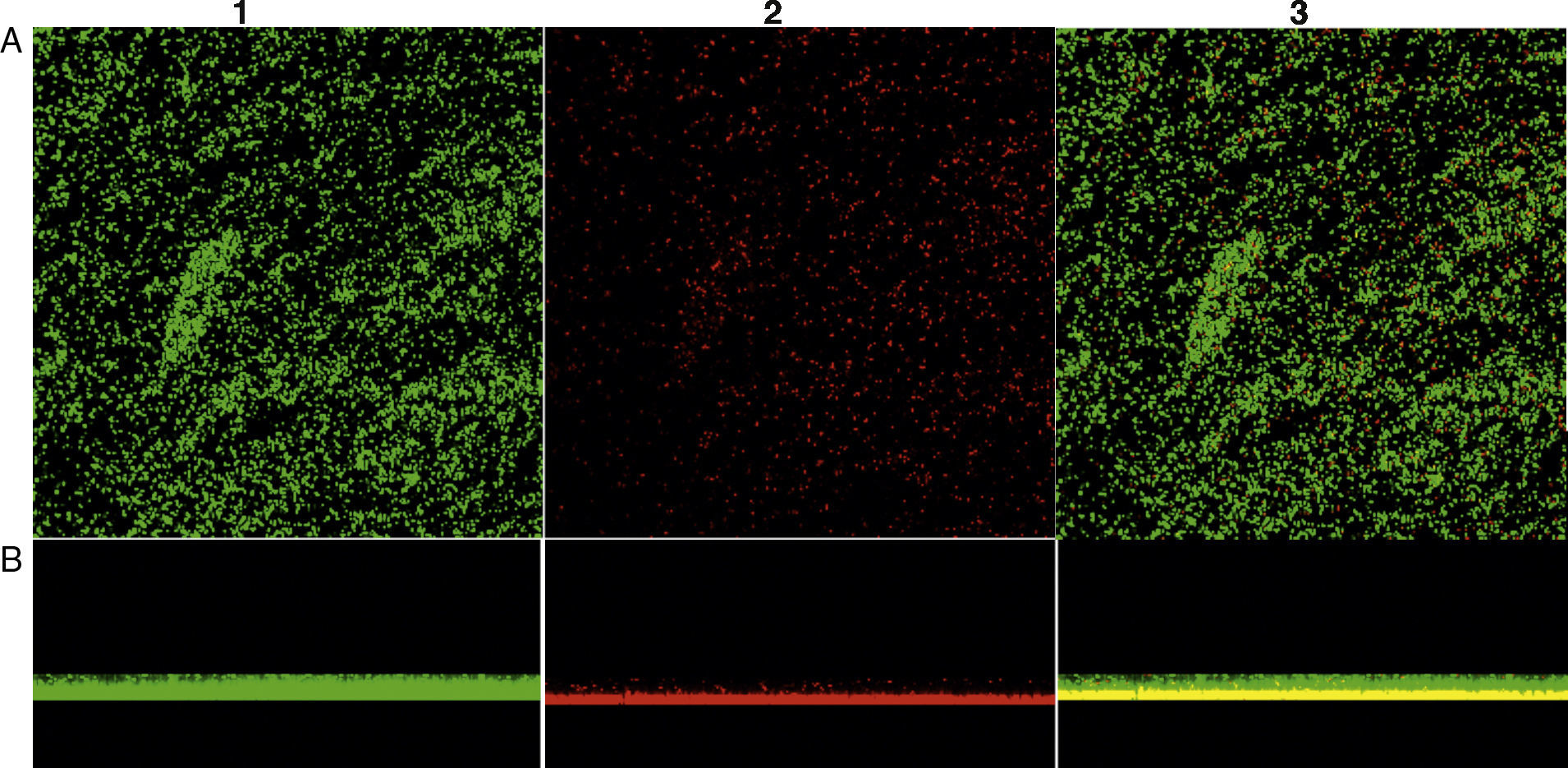
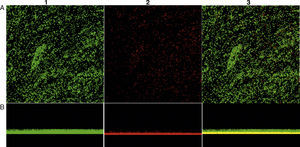
In addition, to confirm the differences between biofilm phenotypes, as determined by BU values, confocal laser scanning microscopy (CLSM) was used to obtain the structural images of the biofilms.13 Here, the biofilm assays were performed at the same way, but after being fixed, the bacterial cells were stained with 25nM SYTO9 and propidium iodide (Live/Dead Bacteria – Invitrogen) for 15min in the dark. The stain was gently removed and biofilms were observed with a CLSM (Carl Zeiss LSM 510, Germany, equipped with Argon laser, 488nm, and 2 helium/neon 543nm wavelengths) to visualize the luminescence of fluochromes.
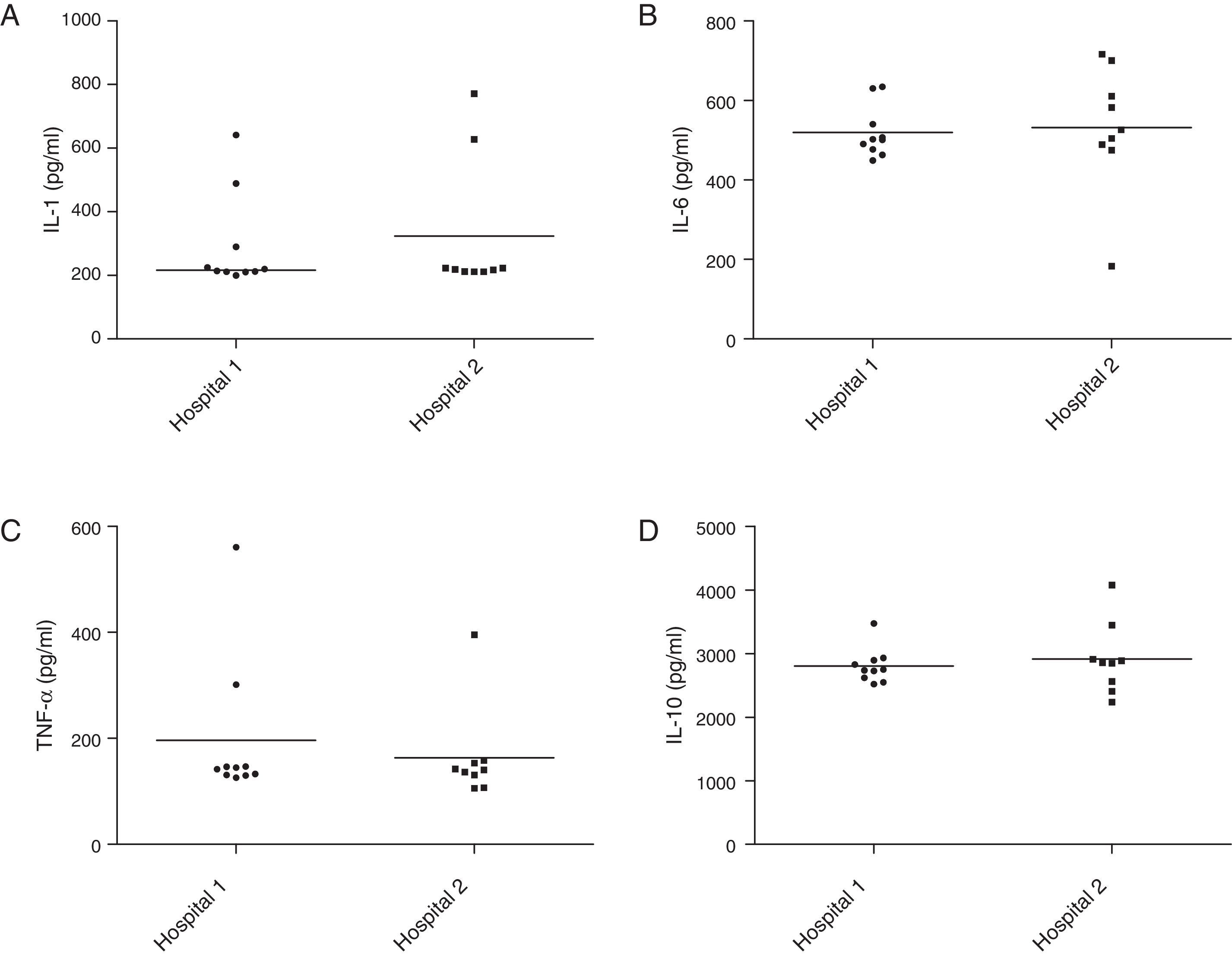
Cytokine induction in murine macrophagesStaphylococcal cells were homogenized in 0.9% sodium chloride solution and the suspensions were adjusted to 0.5×108CFU/mL by spectrophotometer. Then an aliquot of 100mL was mixed with 2mL of Minimum Essential Medium (MEM) with 2mM of l-glutamine and Earle's balanced salts, supplemented with 10% of fetal calf serum (Cult Lab, São Paulo, Brazil), and incubated in a shaker at 250rpm at 35°C for 24h. Subsequently, the cultures were filtrated through 0.22-μm pores. The filtrates were inoculated into J774 murine macrophages. The sets of inoculated cells were incubated at 37°C in 5% CO2 atmosphere for 24h. The supernatants were removed and the cytokines TNF-α, IL-1, IL-6 and IL-10 were measured using ELISA, according to manufacturer instructions (eBioscience, San Diego, CA).
Analysis of efficacy of different disinfectant solutionsThe methodology followed the method of antimicrobial sensitivity of disinfectants recommended by the National Committee for Clinical Laboratory Standards.8 This test was modified to use with a Steers replicator. Disinfecting agents used in the hospitals and chosen for this study included: sodium hypochlorite (0.5, 1.0 and 2.0%); 2.0% glutaraldehyde; 10.0% formaldehyde; ethanol at 70% p/p; 2.0% chlorhexidine gluconate; 2.0% peracetic acid; quaternary ammonium; 100% white vinegar (4% acetic acid). The isolates were placed on Müller-Hinton agar and a Steers applicator was used to apply about 2μL of each disinfectant to certain points on the agar. The contact time of the applicator on the plate was approximately 20–30s. The plates were kept at room temperature to allow the moisture to be absorbed by the agar at the point of application and, after this process, were incubated at 35°C for 24h. We observed the presence or absence of an inhibition zone of visible growth (99.9% of microbial death) on the surface of the agar where the disinfectant agent was applied. All experiments were performed in duplicate with three independent repetitions. The results were analyzed using equality proportions testing with continuity correction (R Project, Vienna, Austria).
Statistical analysisData were analyzed using GraphPad software. A nonparametric test, the Mann–Whitney U test was used to compare continuous variables between hospital 1 and 2 data. Data were considered statistically significant at the p<0.05 level.
ResultsIsolation and antibiotic susceptibilityAfter processing, the study isolated 32 S. aureus from the two public hospitals of Vitória da Conquista: 12 (37.5%) from hospital one and 20 (62.5%) from hospital two. The collection sites providing the highest isolation include: the meeting room, medical clinic and emergency room. The oxacillin susceptibility test was performed with all 32 strains. Bacteria strains showed significant sensitivity to oxacillin. All S. aureus isolated from hospital one were susceptible to oxacillin. Similarly, only two (10%) strains of hospital two showed resistance to oxacillin (Minimum Inhibitory Concentration (MIC)≥4μg/mL). However, these strains were negative to mecA gene amplification by PCR.
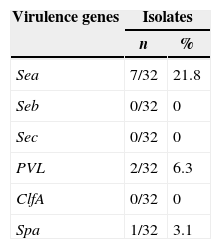
Pathogenic genesRegarding the expression of virulence factors, only Sea, SPA and PVL were detected in 7 (21.8%), 2 (6.3%) and 1 (3.1%) isolates, respectively (Table 1).
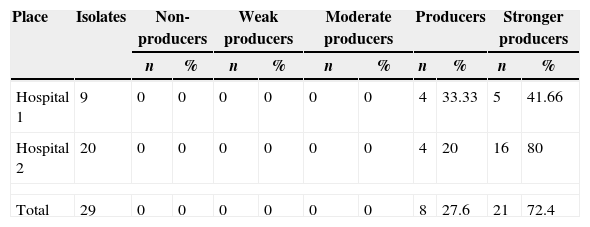
Biofilm productionEvaluation of biofilm production was performed with 29 isolates, 8 (27.58%) classified as biofilm producers and 21 (72.4%) as strong producers. No isolate was classified as biofilm non-producers (Table 2). Fig. 1 shows the biofilm having a thickness of approximately 11μm. There was no statistical difference in biofilm formation among isolates obtained from the two hospitals (p>0.05, Mann–Whitney test, GraphPad Prism) (Fig. 2).
Biofilm production of Staphylococcus aureus carried by insect isolates obtained in two public hospitals of Vitória da Conquista, Bahia, Brazil.
| Place | Isolates | Non-producers | Weak producers | Moderate producers | Producers | Stronger producers | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | ||
| Hospital 1 | 9 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 33.33 | 5 | 41.66 |
| Hospital 2 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 20 | 16 | 80 |
| Total | 29 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 27.6 | 21 | 72.4 |
Confocal microscopy showing biofilm formation of isolated Staphylococcus aureus samples, and (A) shows the top view of the biofilm and (B) the side view of the biofilm. The microorganisms were marked with SYTO9 (green, 1) and unviable with propidium iodide (red, 2). Image 3 is an overlay of images 1 and 2.
Dispersion analysis of samples regarding production of biofilm of Staphylococcus aureus carried by insects isolated in two hospitals of Vitória da Conquista, Bahia, Brazil. As the cutoff point for the production was taken into account, the absorbance obtained by S. pyogenes (O.D.492 0.07). There was no statistical difference in biofilm formation between MRSA and MSSA isolates obtained (p>0.05, Mann–Whitney test, GraphPad Prism®).
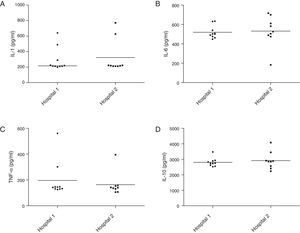
The analysis of cytokine induction in the inflammatory response of J774 macrophages by isolates from the two hospitals did not show statistical difference in the levels of IL-6 (Fig. 3A), TNF-α (3B), IL-1 (3C) and IL-10 (3D) production (p>0.05, Mann–Whitney test, GraphPad Prism®).
Production of cytokines involved in the inflammatory response by Staphylococcus aureus carried by insects isolated in two hospitals of Vitória da Conquista, Bahia, Brazil. There was no statistical difference in IL-6 (A), TNF-α (B), IL-1 (C) and IL-10 (D) induction between samples isolated from two hospitals (p>0.05, Mann–Whitney test, GraphPad Prism®).
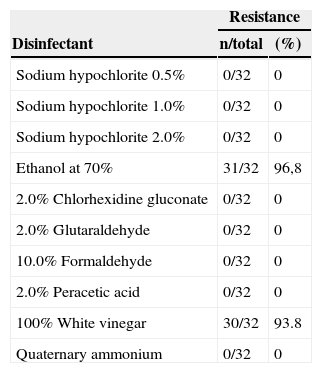
The isolates were used to test disinfectants and antiseptic solutions, with the aim of assessing the effectiveness of these substances within specified conditions. These results are summarized in Table 3. Sodium hypochlorite (0.5–2.0%), 2% chlorhexidine gluconate, quaternary ammonium, peracetic acid and formaldehyde were effective against the isolates tested. The strains showed higher resistance to vinegar (4% acetic acid) (30/32 – 93.8%) and alcohol (31/32 – 96.8%). No differences were observed in disinfectant resistance frequency of isolates from the two hospitals (p>0.05, Mann–Whitney test, GraphPad Prism®).
Analysis of efficacy of different disinfectant solutions against Staphylococcus aureus carried by insects isolated in two public hospitals of Vitoria da Conquista, Bahia.
| Resistance | ||
|---|---|---|
| Disinfectant | n/total | (%) |
| Sodium hypochlorite 0.5% | 0/32 | 0 |
| Sodium hypochlorite 1.0% | 0/32 | 0 |
| Sodium hypochlorite 2.0% | 0/32 | 0 |
| Ethanol at 70% | 31/32 | 96,8 |
| 2.0% Chlorhexidine gluconate | 0/32 | 0 |
| 2.0% Glutaraldehyde | 0/32 | 0 |
| 10.0% Formaldehyde | 0/32 | 0 |
| 2.0% Peracetic acid | 0/32 | 0 |
| 100% White vinegar | 30/32 | 93.8 |
| Quaternary ammonium | 0/32 | 0 |
The presence of insects in homes is occasionally considered a risk to health.14 However, in a hospital environment, they can characterize a definite potential risk to public health. Insects can serve as carriers of pathogenic microorganisms and thus be responsible for severe nosocomial infections.15,16
There are few reports in the literature that consider presence of insects in a hospital environment as mechanical vectors of S. aureus.17 Most studies have focused only on insect species that are susceptible to the bacterial strain.18 Some authors call attention to the potential presence of these insects in the hospital environment as responsible for infections.19 Surveys of ants in Brazilian hospitals show that these insects can carry pathogenic and antibiotic resistant strains.16 These data represent a potential risk of nosocomial infections due to their high mobility within these environments.20,21
In the present study, 32 strains of S. aureus were isolated. Similarly, in a study in a university hospital isolating S. aureus from ants, the authors observed that these insects had a great potential to disperse these bacteria, as well as to acquire microorganisms in contaminated sites and function as a vector in restricted environments, such as a surgical center.15,21 These results were also observed by Pesquero et al.15 who obtained an isolation rate of 13%, whereas S. aureus was the second most frequent pathogen.
Fontana et al.14 isolated S. aureus from 132 ants, and other studies also indicate the isolation of S. aureus from ants collected in hospitals.3 Based on these data we can confirm the hypothesis that certain bacteria are carried by insects and can multiply in their bodies or in the nest and can be a source of infection for immunosuppressed patients within the hospital environment. Several studies have been developed to evaluate the real potential of transmitting pathogens by insects.21 Zarchi and Vatani,22 in a study conducted at a hospital in Havana, Cuba, 19 species of bacteria were isolated from 305 cockroaches. Among them, the isolation rate of S. aureus was 1%.
There are few studies in the literature on biofilm formation by S. aureus isolated from insects. Biofilm formation becomes a major factor for persistent or chronic bacterial infection.23 The microorganisms in a biofilm are more resistant to the action of chemical and physical agents.24 According to Gotz,25 biofilm is a survival strategy in adverse environments, as a result of change of planktonic cells to sessile. In this study, eight (27.6%) were considered strain producers of biofilm, while 21 (72.4%) were considered strong producers. Similar results were obtained by Cramton et al.26 who found 100% of biofilm production in strains of S. aureus isolates from various human infections.
In the present study, some virulence genes were analyzed in isolates. The sea, spa and PVL genes were detected in both staphylococci biotypes, but not the seb, sec and CflA genes. The virulence genes of S. aureus described in the literature show variations. In Brazil, a study27 detected that PVL was rarely present in MRSA and MSSA hospital isolates. Souza and Figueiredo,27 detected the seb gene in three MSSA isolates (3/50) and in four isolates MRSA (4/50), collectively accounting for 3.3% of the total isolates analyzed (7/214). Kim et al.28 observed that none of the MRSA isolates of the SCCmecIII type carried the seb and sec genes. Other authors studying MRSA and MSSA isolates obtained from a university hospital and more frequently detected the genes related to toxins (sea, seb, sed, seg, sei, sej, and eta), and, the pvl, tst and sec genes were more frequent in MSSA.29 Aung et al.6 verified that the MRSA clinical strains had only a few or no staphylococcal enterotoxin (SE) genes, whereas the PVL gene was detected in MSSA and MRSA isolates recovered from a healthy adult possessing an enterotoxin gene cluster (seg, sei, sem, sen, seo, and selu).
In another study, approximately 50% of all isolates produced at least one enterotoxin and 21.5% of the S. aureus isolates from produced PVL. Genes encoding clumping factor B, and elastin and laminin binding proteins were detected in almost all isolates (80%), irrespective of the geographical origin.30 Despite the fact that these genes are carried by mobile genetic elements and, thus, could theoretically be present or absent in different isolates of a specific lineage, the existence of a correlation of a specific clone type and superantigen profiles, in a hospital or in a geographical area, should be investigated in order to trace potential staphylococcal virulence syndrome-associated isolates.
No statistical difference was observed among the studied staphylococcal isolates for the production of inflammatory cytokines. In fact, these compounds are induced mainly by the exocellular lipoteichoic acid of S. aureus.31 In animal models, lipoteichoic acid can induce features of sepsis such as delayed circulatory failure with hypotension and multiple organ failure.32 Jones et al.33 demonstrated that the staphylococcal exocellular lipoteichoic acid is a potent activator of pro-inflammatordy cytokines (TNF-α, IL-6 and IL-1) and nitric oxide in a murine macrophage cell line. The exocellular lipoteichoic acid is significantly more active than that of lipoteichoic acid, peptidoglycan or wall teichoic acid, especially for TNF-α and nitric oxide production. Other virulence factors could be associated with the intense inflammatory response, such as PVL34 or enterotoxin35 but in the present study, the relationship between the presence of these genes and increased production of cytokines was not observed.
Hospital environments are related to infections and may be transmission sources of pathogenic microorganisms, thus emphasizing the importance of hygiene and asepsis in this environment. Due to the high resistance of bacteria to antibiotics, the use of disinfectants with a broad spectrum of action is of great importance, since the elimination of these bacteria would prevent further spreading. In this study, sodium hypochlorite (0.5–2.0%), 2% chlorhexidine gluconate, quaternary ammonium, peracetic acid and formaldehyde were effective against the isolates tested. These results are consistent with findings in the literature.36,37
Although it is almost universally recognized as an effective agent, alcohol use is fraught with controversy and conflicting findings.38 In this study, alcohol was not effective against any of the isolates, and 96.8% of S. aureus strains were resistant to this disinfectant. These results are not consistent with other studies.39 This result may have occurred due to volatilization and thus require time to act efficiently in bacterial protein denaturation. Dos Santos et al.39 observed that the action time influence the low efficiency. The authors report that alcohol lost its effectiveness when used for less than a minute. Pontual et al.40 did not observe the same result. However, the test consisted of immersing the material, and did not offer conditions of evaporation of alcohol. Moreover, other possible reasons, such as biofilm growing microorganisms or modified target sites that tend to be more resistant to the action of chemical and physical agents.
Vinegar (4% acetic acid) was included in the study because it contains acetic acid in its formulation, in order to test its efficacy against clinical isolates of S. aureus. The results showed low efficacy, with only two isolates being sensitive. Rutala and Weber41 and Silva et al.42 also observed low efficacy against S. aureus strains. However, the authors found activity against Gram-negative strains. On the other hand, Utyama43 observed disinfectant efficacy against all strains using white vinegar at a concentration of 3% of acetic acid. These contradictory results suggest that more research is needed to determine optimal uses of vinegar in the hospital routine.
Research on S. aureus carried by insects in hospital environments is very important in relation to the control of nosocomial infections, which are becoming a major challenge. These infections cause high morbidity and mortality and increased hospitalization time having a consequent increase in costs.44 Insects are able to explore various spaces in a hospital environment, making them a potential health risk, due to their ability to disperse pathogenic strains.45 Their growth is facilitated by fluids and food, as well as structural flaws in the hospital environment.14 Hospital infection is a major challenge for health professionals working in this area. The need to control and limit insects has been stressed by most researchers.
Conflicts of interestThe authors declare no conflicts of interest.
This study was supported by FAPESB (N.SUS0012/2009) and PIBIC/CNPq. We thank the hospitals for valuable assistance and AcademicEnglishSolutions.com for proofreading.