To analyze the behavior of meningococcal disease in the Federal District, Brazil, from 2005 to 2011, and to assess the direct impact of the meningococcal serogroup C conjugate vaccine.
MethodsA descriptive study of cases of meningococcal disease among residents of the Federal District. We included in the study confirmed cases of meningococcal disease reported to the local surveillance. To reduce underreporting we compared data to the Brazilian Mortality Database and the Public Health Laboratory Database. We studied sociodemographic, clinical, and pathogen-related variables. For the assessment of the impact of meningococcal serogroup C conjugate vaccine, which was introduced in 2010 for children under two years of age, we compared the incidence of meningococcal disease before and after vaccine introduction in the recommended age groups for vaccination.
ResultsWe identified 309 cases of meningococcal disease, of which 52.1% were males. The average case fatality rate was 20.7%, the median age was three years and there was a predominance of serogroup C (70.2%) and C:23:P1.14-6 phenotype throughout the study period. In 2005–2009, 2010 and 2011, the incidence rates of meningococcal disease were 2.0, 1.8 and 0.8/100,000 inhabitants/year, while mortality rates were 0.4, 0.4 and 0.2/100,000 inhabitants/year, respectively. In the first and last periods, the incidence in poorer and more affluent areas were, respectively, 2.0 and 0.8, and 0.9 and 0.0/100,000 inhabitants/year. Comparing 2009 (the year prior to the introduction of meningococcal serogroup C conjugate vaccine) and 2011, there was 85% reduction in the incidence of serogroup C meningococcal disease in children under four years of age, from 9.0 to 1.3/100,000 (p<0.01).
ConclusionsThe meningococcal serogroup C conjugate vaccine strategy implemented in Brazil proved highly effective and had a strong direct impact on the target population. However, case fatality rates of meningococcal disease remain high with a wide gap in the risk of disease between poor and affluent areas, pointing to the need to reexamine the current strategy on a regular base.
Meningococcal disease (MD) is one of the most feared human infectious diseases, mainly due to its rapid progression and high case fatality rate even with adequate treatment.1
MD has high incidence rates in Brazil and epidemic disease waves have been reported especially of serogroups B and C. The most dramatic epidemic of MD occurred in the early 1970s when there was an overlapping of two epidemic waves, one caused by serogroup A and another caused by serogroup C. This epidemic outbreak reached its peak in 1974 when 179 cases/100,000 inhabitants/year were reported in the state capital of São Paulo.2,3
Brazil has reported epidemics of MD associated with serogroup C since 2005,4,5 with case fatality rates around 20%, high incidence rates among children under two years old (around 8.0/100,000 inhabitants/year) and nearly 40% of the cases occurring in children under four years old.5 In 2010, Brazilian health authorities introduced routine vaccination with the meningococcal serogroup C conjugate vaccine (MCCV).6 However, unlike other countries, Brazil has implemented a vaccination strategy targeting only children under two years of age, but no mass vaccination of adolescents.7,8
Given the MD burden in Brazil5 and to better understand the impact of the vaccination strategy implemented, we conducted a study to examine MD occurrence in the Federal District from 2005 to 2011 and to assess the direct impact of the introduction of MCCV on the targeted age group for vaccination.
Materials and methodsThis is a descriptive study conducted in the Federal District of Brazil, which is located in the central-west region of the country and is divided into 30 administrative areas9,10 with an estimated population of 2,600,000 people (2011). Although it has the ninth highest human development index (HDI) in Brazil (0.824),11 there are significant social disparities among administrative areas. The illiteracy rate among people older than 15 years ranges between 0.6% and 8.0%; the proportion of households served by sewage treatment systems ranges from 4.2% to 91.8%; and the average monthly per capita income varies from 0.4 to 10.8 minimum wages.12
Confirmed case of MD was defined as a person residing in the Federal District with symptom onset between January 1, 2005 and December 31, 2011 and clinical manifestations consistent with MD who met at least one of the following criteria: isolation of Neisseria meningitidis from the cerebrospinal fluid (CSF) or blood; positive immunodiagnostic test for N. meningitidis antigen in the CSF or blood; presence of Gram-negative diplococcus in the CSF; or association or not of purpura fulminans with meningitis. All cases that met the inclusion criteria were included in the study.
We studied sociodemographic (gender, age, area of residence), clinical (clinical presentation, progression, and time [in days] from symptom onset to discharge/death; diagnostic criterion and time [in days] from symptom onset to hospitalization), and pathogen-related variables (serogroup, serotype and serosubtype) and time of disease occurrence (month and year).
MD passive surveillance data were obtained from the National Notifiable Disease Database (SINAN); diagnostic data from the Central Public Health Laboratory of the Federal District (LACEN-DF) and Instituto Adolfo Lutz of São Paulo (IAL)/National Reference Center for Meningitis; mortality data from the National Mortality Database (SIM); and population data from the Brazilian Institute of Geography and Statistics (IBGE).9
For creating a database, we used data from SINAN and performed consistency analysis and duplicate elimination. We then compared them to data from the LACEN-DF/IAL database to address missing data. Our prior database was then manually linked with deaths reported in the SIM database for the study period coded A39.0 (meningococcal meningitis) and A39.2 (acute meningococcemia).13 Deaths that were found only in the SIM database were included in the study database. Cases that had missing information on progression and were not in the SIM database were considered as cure.
Data analysisWe first conducted a descriptive analysis to describe cases according to the variables of interest. Categorical variables were compared using the chi-square test.
For the estimation of incidence and mortality rates, we used confirmed cases of MD and deaths from MD as numerator, respectively, and the estimated population at the midpoint of the study period as denominator. For the calculation of the average annual incidence and mortality rates, we divided the period incidence or period mortality rate of MD by the number of years in that period. For the estimation of case fatality rates, we used the total number of deaths from MD as numerator and the total number of confirmed cases of MD as denominator.
The MCCV was introduced in the Federal District in August 2010. From September to December 2010, the vaccine was recommended for children between three months and two years of age. Since 2011, the vaccine schedule changed to children under one year of age, with two doses of vaccine given by three and five months of age, and a booster dose by 12 months. For the assessment of the direct impact of MCCV, we estimated total incidence of MD and by age group for the years 2009 and 2011. We also compared incidence rates of serogroups B and C before and after vaccine introduction, by age group. The analyses were conducted with the use of SPSS version 20 and Microsoft Office Excel® 2007.
This study was approved by the Research Ethics Committees of the Health Department of the Federal District (protocol number 373/11) and the Universidade de São Paulo School of Public Health (Plataforma Brasil – CAAE: 01431312.8.0000.5421).
ResultsFrom 2005 to 2011, we identified 309 cases of MD that met the study inclusion criteria. Of these, 178 (57.6%) were confirmed by culture, 94 (30.4%) by a clinical criterion, 18 (5.8%) by latex test, 17 (5.5%) by bacteroscopic examination, and 2 (0.6%) by counterimmunoelectrophoresis.
Median time from symptom onset to hospitalization was one day (ranging from less than 24h to five days) and median time from symptom onset to the outcome (discharge/death) was 10 days (ranging from less than 24h to 123 days). These data refer to cases with information available, i.e., 274 and 266 of the 309 cases studied. The most common clinical presentations of MD were meningococcal meningitis (35.1%; 100/285) and meningococcal meningitis with meningococcemia (35.8%; 102/285).
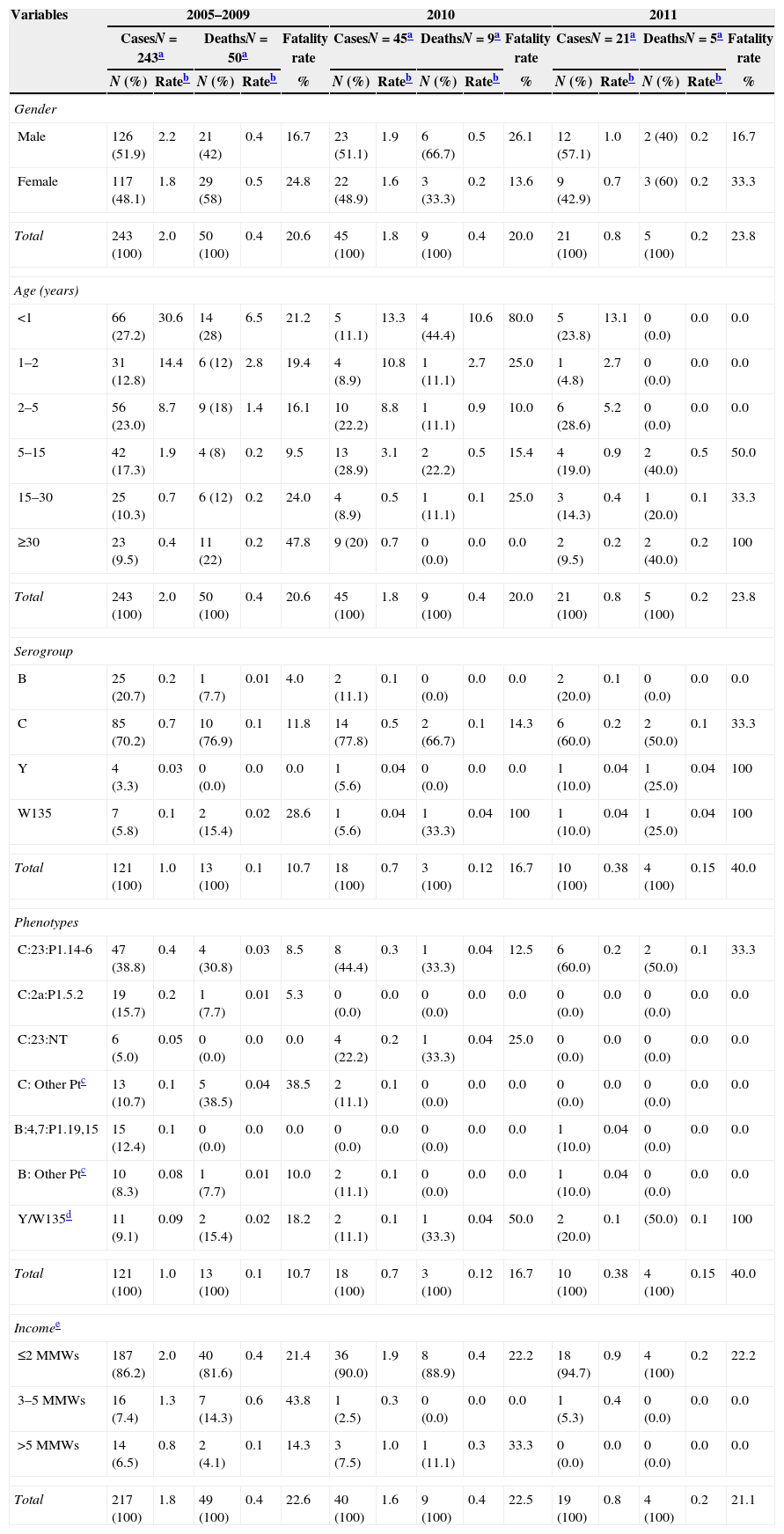
Of the 309 individuals with MD, 52.1% were male. From 2005 to 2009, 40.0% of cases of MD occurred in children under two and 23% were two to four years of age; in 2010, these proportions were 20% and 22.2%, and in 2011, 28.6% and 28.6% (Table 1). The median age in the three time periods (2005–2009, 2010 and 2011) were three years (range: nine days to 78 years), five years (range: one month to 66 years), and four years (range: 3 months to 77 years), respectively.
Incidence, mortality and case fatality rates of meningococcal disease at different times according to sociodemographic and pathogen-related variables. Federal District, Brazil.
| Variables | 2005–2009 | 2010 | 2011 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CasesN=243a | DeathsN=50a | Fatality rate | CasesN=45a | DeathsN=9a | Fatality rate | CasesN=21a | DeathsN=5a | Fatality rate | |||||||
| N (%) | Rateb | N (%) | Rateb | % | N (%) | Rateb | N (%) | Rateb | % | N (%) | Rateb | N (%) | Rateb | % | |
| Gender | |||||||||||||||
| Male | 126 (51.9) | 2.2 | 21 (42) | 0.4 | 16.7 | 23 (51.1) | 1.9 | 6 (66.7) | 0.5 | 26.1 | 12 (57.1) | 1.0 | 2 (40) | 0.2 | 16.7 |
| Female | 117 (48.1) | 1.8 | 29 (58) | 0.5 | 24.8 | 22 (48.9) | 1.6 | 3 (33.3) | 0.2 | 13.6 | 9 (42.9) | 0.7 | 3 (60) | 0.2 | 33.3 |
| Total | 243 (100) | 2.0 | 50 (100) | 0.4 | 20.6 | 45 (100) | 1.8 | 9 (100) | 0.4 | 20.0 | 21 (100) | 0.8 | 5 (100) | 0.2 | 23.8 |
| Age (years) | |||||||||||||||
| <1 | 66 (27.2) | 30.6 | 14 (28) | 6.5 | 21.2 | 5 (11.1) | 13.3 | 4 (44.4) | 10.6 | 80.0 | 5 (23.8) | 13.1 | 0 (0.0) | 0.0 | 0.0 |
| 1–2 | 31 (12.8) | 14.4 | 6 (12) | 2.8 | 19.4 | 4 (8.9) | 10.8 | 1 (11.1) | 2.7 | 25.0 | 1 (4.8) | 2.7 | 0 (0.0) | 0.0 | 0.0 |
| 2–5 | 56 (23.0) | 8.7 | 9 (18) | 1.4 | 16.1 | 10 (22.2) | 8.8 | 1 (11.1) | 0.9 | 10.0 | 6 (28.6) | 5.2 | 0 (0.0) | 0.0 | 0.0 |
| 5–15 | 42 (17.3) | 1.9 | 4 (8) | 0.2 | 9.5 | 13 (28.9) | 3.1 | 2 (22.2) | 0.5 | 15.4 | 4 (19.0) | 0.9 | 2 (40.0) | 0.5 | 50.0 |
| 15–30 | 25 (10.3) | 0.7 | 6 (12) | 0.2 | 24.0 | 4 (8.9) | 0.5 | 1 (11.1) | 0.1 | 25.0 | 3 (14.3) | 0.4 | 1 (20.0) | 0.1 | 33.3 |
| ≥30 | 23 (9.5) | 0.4 | 11 (22) | 0.2 | 47.8 | 9 (20) | 0.7 | 0 (0.0) | 0.0 | 0.0 | 2 (9.5) | 0.2 | 2 (40.0) | 0.2 | 100 |
| Total | 243 (100) | 2.0 | 50 (100) | 0.4 | 20.6 | 45 (100) | 1.8 | 9 (100) | 0.4 | 20.0 | 21 (100) | 0.8 | 5 (100) | 0.2 | 23.8 |
| Serogroup | |||||||||||||||
| B | 25 (20.7) | 0.2 | 1 (7.7) | 0.01 | 4.0 | 2 (11.1) | 0.1 | 0 (0.0) | 0.0 | 0.0 | 2 (20.0) | 0.1 | 0 (0.0) | 0.0 | 0.0 |
| C | 85 (70.2) | 0.7 | 10 (76.9) | 0.1 | 11.8 | 14 (77.8) | 0.5 | 2 (66.7) | 0.1 | 14.3 | 6 (60.0) | 0.2 | 2 (50.0) | 0.1 | 33.3 |
| Y | 4 (3.3) | 0.03 | 0 (0.0) | 0.0 | 0.0 | 1 (5.6) | 0.04 | 0 (0.0) | 0.0 | 0.0 | 1 (10.0) | 0.04 | 1 (25.0) | 0.04 | 100 |
| W135 | 7 (5.8) | 0.1 | 2 (15.4) | 0.02 | 28.6 | 1 (5.6) | 0.04 | 1 (33.3) | 0.04 | 100 | 1 (10.0) | 0.04 | 1 (25.0) | 0.04 | 100 |
| Total | 121 (100) | 1.0 | 13 (100) | 0.1 | 10.7 | 18 (100) | 0.7 | 3 (100) | 0.12 | 16.7 | 10 (100) | 0.38 | 4 (100) | 0.15 | 40.0 |
| Phenotypes | |||||||||||||||
| C:23:P1.14-6 | 47 (38.8) | 0.4 | 4 (30.8) | 0.03 | 8.5 | 8 (44.4) | 0.3 | 1 (33.3) | 0.04 | 12.5 | 6 (60.0) | 0.2 | 2 (50.0) | 0.1 | 33.3 |
| C:2a:P1.5.2 | 19 (15.7) | 0.2 | 1 (7.7) | 0.01 | 5.3 | 0 (0.0) | 0.0 | 0 (0.0) | 0.0 | 0.0 | 0 (0.0) | 0.0 | 0 (0.0) | 0.0 | 0.0 |
| C:23:NT | 6 (5.0) | 0.05 | 0 (0.0) | 0.0 | 0.0 | 4 (22.2) | 0.2 | 1 (33.3) | 0.04 | 25.0 | 0 (0.0) | 0.0 | 0 (0.0) | 0.0 | 0.0 |
| C: Other Ptc | 13 (10.7) | 0.1 | 5 (38.5) | 0.04 | 38.5 | 2 (11.1) | 0.1 | 0 (0.0) | 0.0 | 0.0 | 0 (0.0) | 0.0 | 0 (0.0) | 0.0 | 0.0 |
| B:4,7:P1.19,15 | 15 (12.4) | 0.1 | 0 (0.0) | 0.0 | 0.0 | 0 (0.0) | 0.0 | 0 (0.0) | 0.0 | 0.0 | 1 (10.0) | 0.04 | 0 (0.0) | 0.0 | 0.0 |
| B: Other Ptc | 10 (8.3) | 0.08 | 1 (7.7) | 0.01 | 10.0 | 2 (11.1) | 0.1 | 0 (0.0) | 0.0 | 0.0 | 1 (10.0) | 0.04 | 0 (0.0) | 0.0 | 0.0 |
| Y/W135d | 11 (9.1) | 0.09 | 2 (15.4) | 0.02 | 18.2 | 2 (11.1) | 0.1 | 1 (33.3) | 0.04 | 50.0 | 2 (20.0) | 0.1 | (50.0) | 0.1 | 100 |
| Total | 121 (100) | 1.0 | 13 (100) | 0.1 | 10.7 | 18 (100) | 0.7 | 3 (100) | 0.12 | 16.7 | 10 (100) | 0.38 | 4 (100) | 0.15 | 40.0 |
| Incomee | |||||||||||||||
| ≤2 MMWs | 187 (86.2) | 2.0 | 40 (81.6) | 0.4 | 21.4 | 36 (90.0) | 1.9 | 8 (88.9) | 0.4 | 22.2 | 18 (94.7) | 0.9 | 4 (100) | 0.2 | 22.2 |
| 3–5 MMWs | 16 (7.4) | 1.3 | 7 (14.3) | 0.6 | 43.8 | 1 (2.5) | 0.3 | 0 (0.0) | 0.0 | 0.0 | 1 (5.3) | 0.4 | 0 (0.0) | 0.0 | 0.0 |
| >5 MMWs | 14 (6.5) | 0.8 | 2 (4.1) | 0.1 | 14.3 | 3 (7.5) | 1.0 | 1 (11.1) | 0.3 | 33.3 | 0 (0.0) | 0.0 | 0 (0.0) | 0.0 | 0.0 |
| Total | 217 (100) | 1.8 | 49 (100) | 0.4 | 22.6 | 40 (100) | 1.6 | 9 (100) | 0.4 | 22.5 | 19 (100) | 0.8 | 4 (100) | 0.2 | 21.1 |
The difference between the total number of cases studied in each period and the total number of cases analyzed in each variable expresses the cases without information.
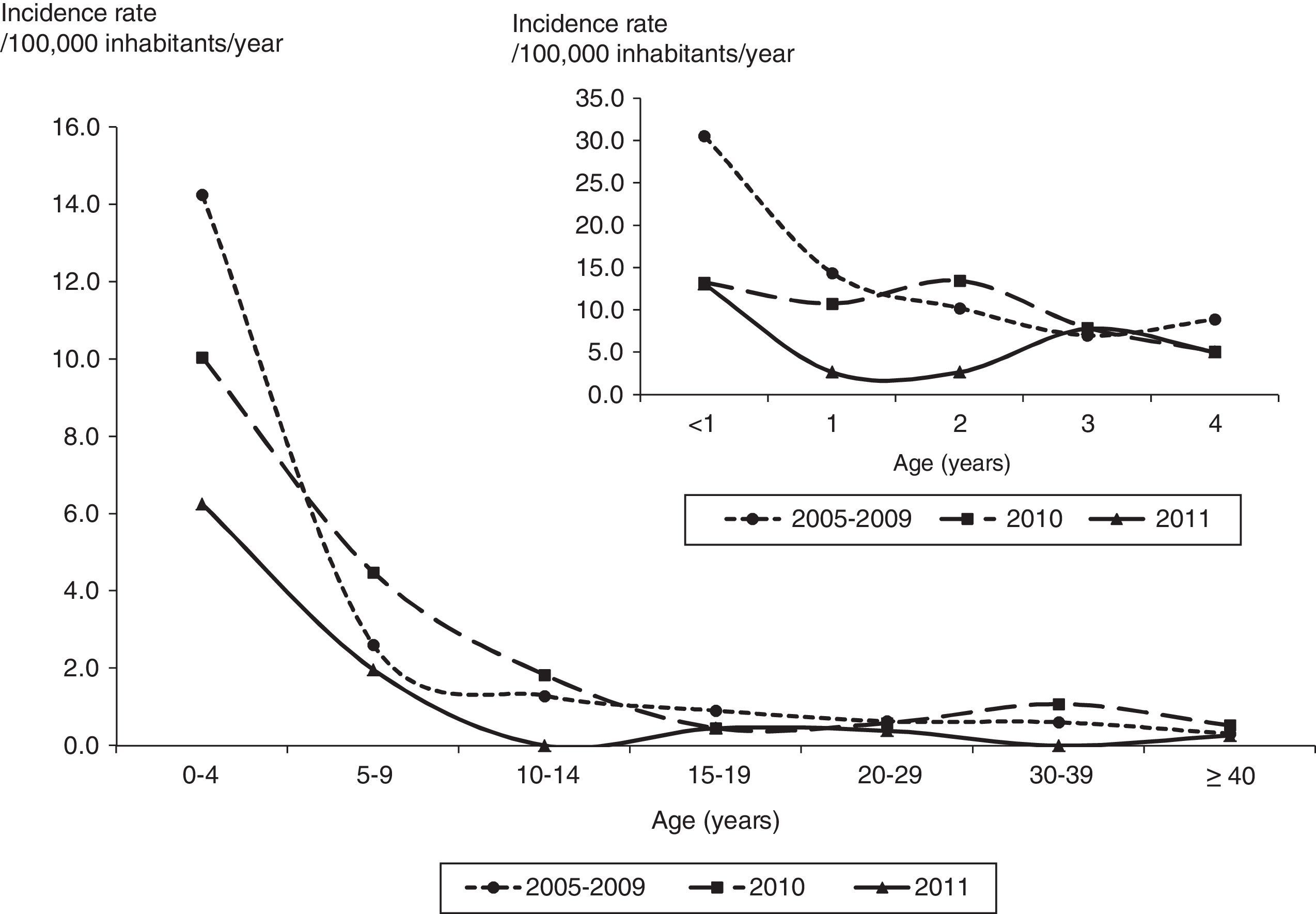
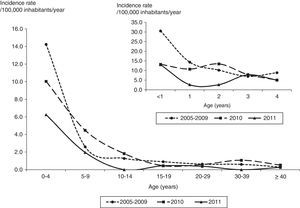
The average annual incidence rate was 2.0/100,000 inhabitants/year (range: 1.3–2.5 per 100,000) from 2005 to 2009 and 1.8 and 0.8/100,000 inhabitants/year in 2010 and 2011. The average annual incidence rates in children under one year of age were 30.6, 13.3 and 13.1/100,000 inhabitants/year and in children with two years of age were 14.4, 10.8 and 2.7/100,000 inhabitants/year from 2005 to 2009 and in 2010 and 2011, respectively (Fig. 1).
The average annual mortality rate was 0.4 (range: 0.3–0.5) from 2005 to 2009, 0.4 in 2010 and 0.2/100,000 inhabitants/year in 2011. The average annual mortality rate in children under one year and one year of age were 6.5 and 2.8/100,000 inhabitants/year, respectively, from 2005 to 2009. In 2011, no deaths in children under five years of age were reported (Table 1).
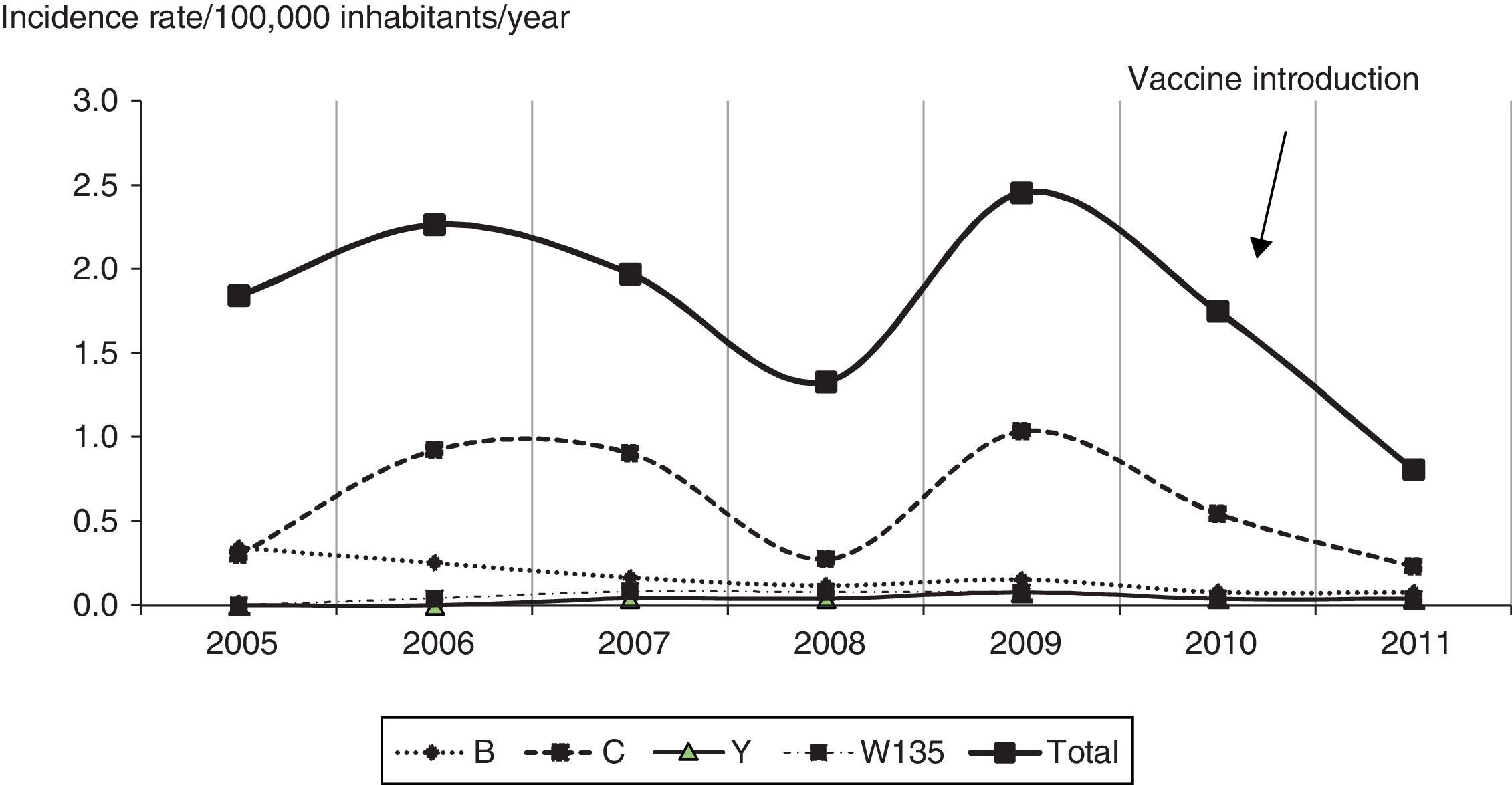
Of cases with information available for serogroup, 70.2% (85/121) were serogroup C from 2005 to 2009, 77.8% (14/18) in 2010, and 60.0% (6/10) in 2011 (Table 1). The incidence rates of serogroup C infection ranged from 0.2 to 1.0/100,000 inhabitants/year (Fig. 2). During the study period (2005–2011), serogroup B infection accounted for 19.5% (29/149) of cases, with decreasing incidence of cases associated with this serogroup over time. Serogroups Y and W135 accounted for 4.1% (6/149) and 6.1% (9/149) of cases. The most common phenotype of serogroup C was C:23:P1.14-6 in the three time points studied (Table 1).
The average case fatality rate for the period 2005–2011 was 20.7%, with no significant variation. However, these rates were not homogeneous among the hospitals treating cases of MD. Of 25 hospitals, a subset of 13 hospitals showed an average case fatality rate of 38.1% and treated no more than three cases during the study period; of these, five were public hospitals; and three had neonatal, pediatric and adult intensive care units (ICUs). Another subset of nine hospitals showed an average case fatality rate of 21.7% and treated six to 18 cases during the study period; of these, seven were public hospitals; and three had ICUs. The three remaining hospitals showed an average case fatality rate of 15.3%; they provided care to 45–58 cases; all of them were public hospitals; and two had ICUs. A comparison of the three subsets of hospitals showed that case fatality rates decreased as the average number of cases treated in a hospital, the number of public hospitals and availability of ICUs increased. These differences were statistically significant (p=0.05; chi square for trend).
The incidence of MD varied by administrative area of residence according to the average per capita income (in monthly minimum wages, MMWs). From 2005 to 2009, the incidence of MD was 2.5 times greater in those living in areas with per capita income of up to two MMWs (1 MMW=US$ 200) than in those residing in areas with income of more than five MMWs (p<0.001). In 2011, the incidence of MD declined by 50% among those living in areas with per capita income of up to two MMWs and no cases of MD were reported among the most affluent population (Table 1). Residents of areas of low per capita income accounted for 73.9% of the Federal District population, while those of middle income with three to five MMWs and more than five MMWs accounted for 10.2% and 12.4% of the population, respectively. Information about this indicator was available for 26/30 administrative areas.
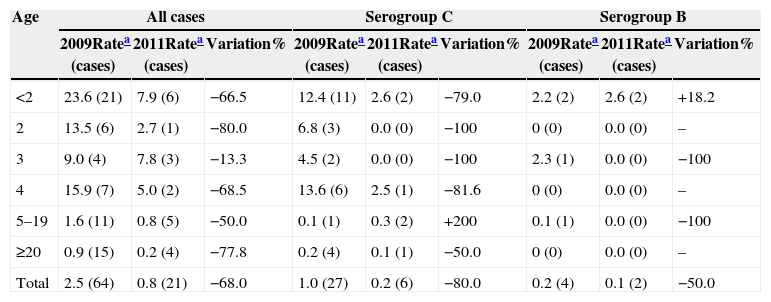
To assess the potential impact of MCCV, we compared incidence rates before (2009) and after (2011) vaccine introduction. There was a 68% decline in overall incidence of MD, i.e., from 2.5 to 0.8/100,000 inhabitants/year (p<0.001). During this same time period, the incidence of MD in children under two and children aged two years fell from 23.6 to 7.9 (66.5%; p=0.02) and from 13.5 to 2.7/100,000 inhabitants/year (80.0%; p=0.19), respectively (Table 2).
Incidence rates before and after the introduction of the meningococcal serogroup C conjugate vaccine. Federal District, Brazil.
| Age | All cases | Serogroup C | Serogroup B | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2009Ratea (cases) | 2011Ratea (cases) | Variation% | 2009Ratea (cases) | 2011Ratea (cases) | Variation% | 2009Ratea (cases) | 2011Ratea (cases) | Variation% | |
| <2 | 23.6 (21) | 7.9 (6) | −66.5 | 12.4 (11) | 2.6 (2) | −79.0 | 2.2 (2) | 2.6 (2) | +18.2 |
| 2 | 13.5 (6) | 2.7 (1) | −80.0 | 6.8 (3) | 0.0 (0) | −100 | 0 (0) | 0.0 (0) | – |
| 3 | 9.0 (4) | 7.8 (3) | −13.3 | 4.5 (2) | 0.0 (0) | −100 | 2.3 (1) | 0.0 (0) | −100 |
| 4 | 15.9 (7) | 5.0 (2) | −68.5 | 13.6 (6) | 2.5 (1) | −81.6 | 0 (0) | 0.0 (0) | – |
| 5–19 | 1.6 (11) | 0.8 (5) | −50.0 | 0.1 (1) | 0.3 (2) | +200 | 0.1 (1) | 0.0 (0) | −100 |
| ≥20 | 0.9 (15) | 0.2 (4) | −77.8 | 0.2 (4) | 0.1 (1) | −50.0 | 0 (0) | 0.0 (0) | – |
| Total | 2.5 (64) | 0.8 (21) | −68.0 | 1.0 (27) | 0.2 (6) | −80.0 | 0.2 (4) | 0.1 (2) | −50.0 |
In addition, during this same time period, there was a significant reduction of 80% in total cases of MD due to serogroup C, i.e., from 1.0 to 0.2/100,000 inhabitants/year (p<0.001). The incidence rates in children under two and children aged two years fell from 12.4 to 2.6 (79.0%) and from 6.8 to zero (100%), respectively (Table 2). The overall reduction in MD cases by serogroup C among children under four years of age was 85.6%, from 9.0 to 1.3/100,000 inhabitants/year (p<0.01). A similar reduction (81.6%) was seen among those aged four years (p=0.17).
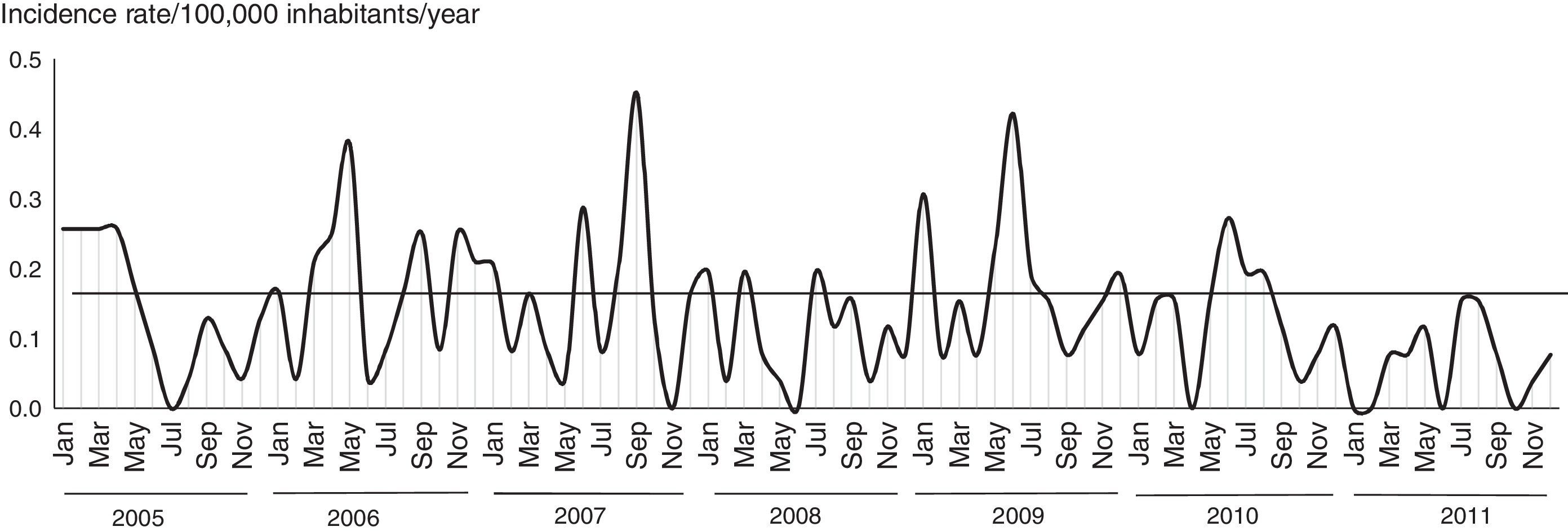
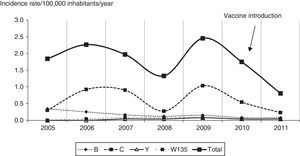
Following vaccine introduction monthly incidence rates of MD did not exceed 0.15/100,000 inhabitants/year (Fig. 3). The incidence rates remained at a lower level than that reported prior to the beginning of the serogroup C epidemic, but with the typical seasonal pattern of MD.
DiscussionThe study results showed that MD was a major public health problem in the Federal District due to its morbidity and mortality especially before the introduction of MCCV. The extent of MD in a community has been strongly associated with local living conditions,14 so these results were unexpected considering that the Federal District has one of the highest HDIs in Brazil.11 However, they may be largely explained by social inequalities; it was evidenced that incidence rates of MD in poor areas were 2.5 times higher than those reported in more affluent areas. These risk differences persisted though at lower levels even after vaccine introduction.
The average annual incidence rate of MD in the Federal District before vaccine introduction was slightly greater than the national average5 and even five times greater than those reported in other Latin American countries such as Argentina, Chile, and Venezuela.15
Children under two years of age showed a high risk of MD, which is consistent with that reported in the literature regardless of the level of development of the country.16–18 This finding is also in agreement with the fact that MD was the third leading cause of death from infectious and parasitic diseases and the ninth leading cause of hospitalization among children under five years of age in the Federal District from 2005 to 2011.19
The study showed that children aged under two years are at higher risk of death from MD, which is corroborated by the literature.20 However, we found lower rates than other Brazilian studies.14,16
The case fatality rate of MD in the Federal District was similar to the average rate nationwide and in some Brazilian capitals14,20 but it was two times higher than that reported in developed countries and other South American countries.15 Some authors have argued that it may be due to difficulties to ensure uniformity in the quality of medical care.21–23 This is further supported by the fact that case fatality rate of MD, in our study, varied among hospitals and was associated with average number of cases treated in a given hospital, type of hospital (public or private), and availability of ICU.
Similar to that seen in other Brazilian regions,5 we also identified during the study period an epidemic wave of MD due to serogroup C that accounted for more than two-thirds of the cases reported. This epidemic wave was predominantly associated with two main phenotypes: C:23:P1.14-6 (most cases) and C:2a:P1.5,2.4,24
Serogroup B was the second major cause of MD during the study period though it showed a steady decline parallel to that seen nationwide.5 Serogroups Y and W135 should also be mentioned. Serogroup Y is an important cause of MD in North America,25 while serogroup W135 is more relevant to our scenario because it showed greater proportion in the Federal District than nationwide average5 and had a significantly higher case fatality rate than other serogroups.26–28 In Chile, serogroup W135 is causing an epidemic of MD since 2012 with a case fatality of around 25%, which is 2.5 times higher than the national average fatality rate before this epidemic.29
With regard to the potential direct impact of MCCV, the analysis evidenced a two-third reduction of the overall incidence of MD, 80% reduction in total cases of MD due to serogroup C and 85% reduction in cases due to serogroup C in cohorts exposed to vaccine. These results strongly support the effectiveness of the proposed vaccination schedule.
The impact seen on children belonging to the age group targeted for vaccination in the Federal District is similar to that reported in the United Kingdom, although their strategy covered individuals aged five months to 18 years.7 In Spain, on the other hand, a greater reduction (around 95%)30 was achieved with vaccination of children aged under five years but significant loss of vaccine-induced immunity was evidenced over time.30
The potential indirect impact of MCCV in children aged four years requires further investigation and longer follow-up. In our study, we identified a reduction of around 81% in these children. The indirect impact of this vaccine was only observed in countries that implemented routine vaccination of children in their first year of life combined with mass vaccination campaigns targeting individuals up to 20 years of age.7,8,31,32 The indirect protection effect from MCCV given to adolescents takes place through eliminating meningococci from carriers that may expose household contacts.33,34
The study results should be interpreted considering some limitations. One limitation is the use of secondary sources of data, which may show inconsistencies of the variables of interest and underreporting. A second limitation is that there may have been bias toward reporting more severe cases, which may have overestimated fatality rates. Another potential limitation of this study is the short post-vaccination time analyzed which may have prevented a more consistent assessment of vaccine impact. Despite these limitations, our results corroborate the literature and provide valuable input to improve control strategies of MD.
In conclusion, the MCCV strategy implemented in Brazil proved highly effective and had a strong direct impact on the target population. However, incidence and case fatality rates of MD remain high with a wide gap in the risk of disease between poor and affluent areas pointing to the need for periodic adjustments and revaluations of the current strategy.
Author contributionsMCT and EAW designed the study, analyzed the data and wrote the manuscript; CSRC and ACV critically revised the manuscript.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Ana Paula Lemos from Instituto Adolfo Lutz and the National Council for Scientific and Technological Development (CNPq) for their support (number: 132840/2011-1).