The present case-report describes tuberculosis on the oral mucosa, in a rare manifestation of the disease. The importance of appropriate diagnosis and awareness of the clinical manifestations is highlighted. Oral lesions seem to occur as chronic ulcers, nodular or granular areas, and rare, firm leukoplakia regions. Most extra-pulmonary lesions represent secondary infections of a primary lung infectious focus; therefore, early and accurate diagnosis is required for planning of the best treatment and strategies to control the disease.
Tuberculosis (TB) is a chronic infectious disease caused by Mycobacterium tuberculosis. Most often it affects the lungs, although some patients present the disease in other organs and systems. Extra-pulmonary TB accounts for 25% of the cases with 10–35% detected in the head and neck region.1,2 Oral manifestation of TB may affect people of all ages, especially the elderly, and is usually presented as an ulcer. It has been hypothesized that autoinoculation may happen when the infected pulmonary mucus interacts with wounded, susceptible areas of the mucosa, eliciting the emergence of lesions.3 The present case-report describes oral manifestation of TB in an adult patient.
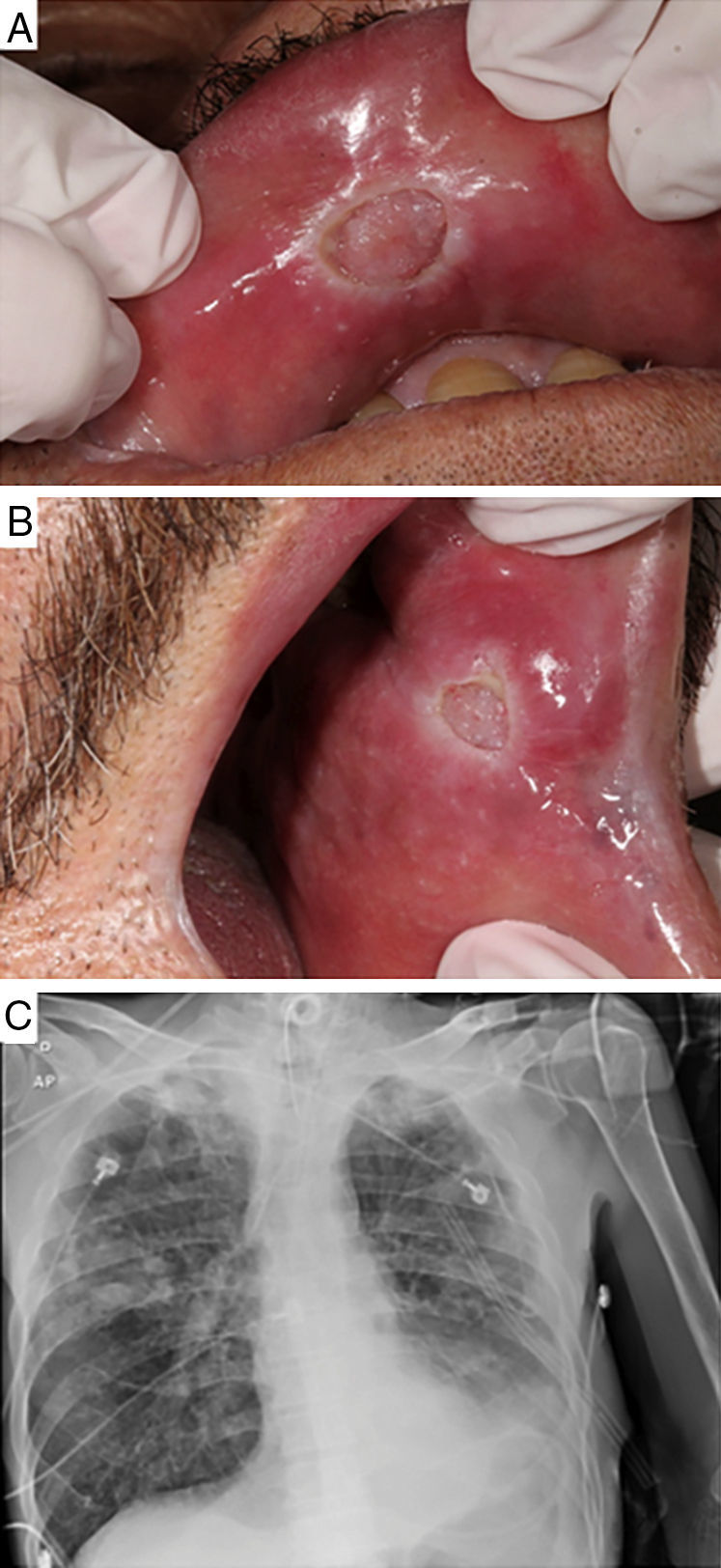
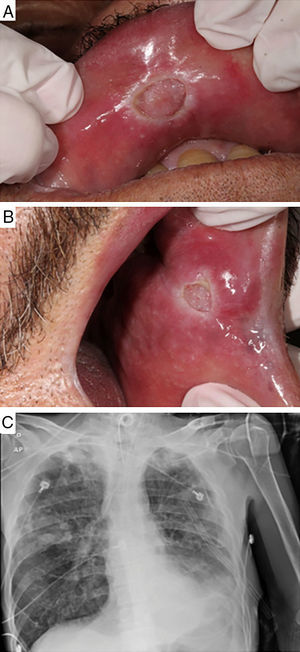
Case-reportA 61-year-old male patient with a history of smoking habit and alcohol abuse was being followed up for uncontrolled type 2 diabetes mellitus, peripheral neuropathy associated with vasculopathy, systemic hypertension, and chronic pancreatitis in Hospital de Clínicas de Porto Alegre (HCPA), state of Rio Grande do Sul, Brazil. The patient was referred to the Stomatology Unit of the same hospital due to the emergence of lesions on the oral mucosa. Preliminary examination revealed two lesions, each measuring approximately 10mm across and presenting a granulomatous central portion and whitish halo. The lesions were located on the upper lip mucosa near the median line and on the left jugal mucosa adjacent to the labial anterior commissure (Fig. 1A and B, respectively). The patient complained of pain, productive cough for the past 15 days, night sweats, episodic fever in the morning, and slight weight loss in the previous two months. However, these complaints were intermittently made by the patient along the scheduled appointments, which may have added to the difficulty for an early diagnosis of the disease. Samples of lesions were collected by incision and stained according to the hematoxylin–eosin (HE) and Ziehl–Neelsen (BAAR) protocols. The pathological report was negative for the presence of alcohol–acid resistant microorganisms (Fig. 2). Due to the comparatively low count of microorganisms in the tissues analyzed, the special staining used did not successfully detect the presence of the bacterium. However, since a negative result in this kind of analysis does not rule out TB, a sample of bronchoalveolar lavage was analyzed according to the Ziehl–Neelsen (BAAR) protocol, with a positive result for M. tuberculosis. A radiograph of the thorax was suggestive of presence of active infectious disease, manifested as budding tree-like centrilobular nodules in both lungs, especially on the right. In this case report, the pathological analysis of the samples collected from the patient was not conclusive, requiring a Mantoux assay and the investigation of bronchoalveolar lavage in order to confirm the TB suspicion. The Mantoux protocol indicated a 13-mm inflammatory reaction confirming TB. Anti-HIV test was negative. The patient was referred to a pulmonologist for examination and treatment based on the pulmonary extension of the disease (Fig. 1C). Nonetheless, 30 days into the treatment the patient died due to the worsening of clinical conditions, sepsis, respiratory failure, and acute kidney failure.
Clinical aspects of oral TB lesions and lung radiographic findings. Ulcerative lesions with granulomatous center and whitish halo on the upper labial mucosa near the median line (A) and on the left jugal mucosa, near the labial posterior commissure (B). Full radiograph of the lower left pulmonary lobe. Presence of active disease manifested as budding tree-like centrilobular nodules in both lungs, especially on the right (C).
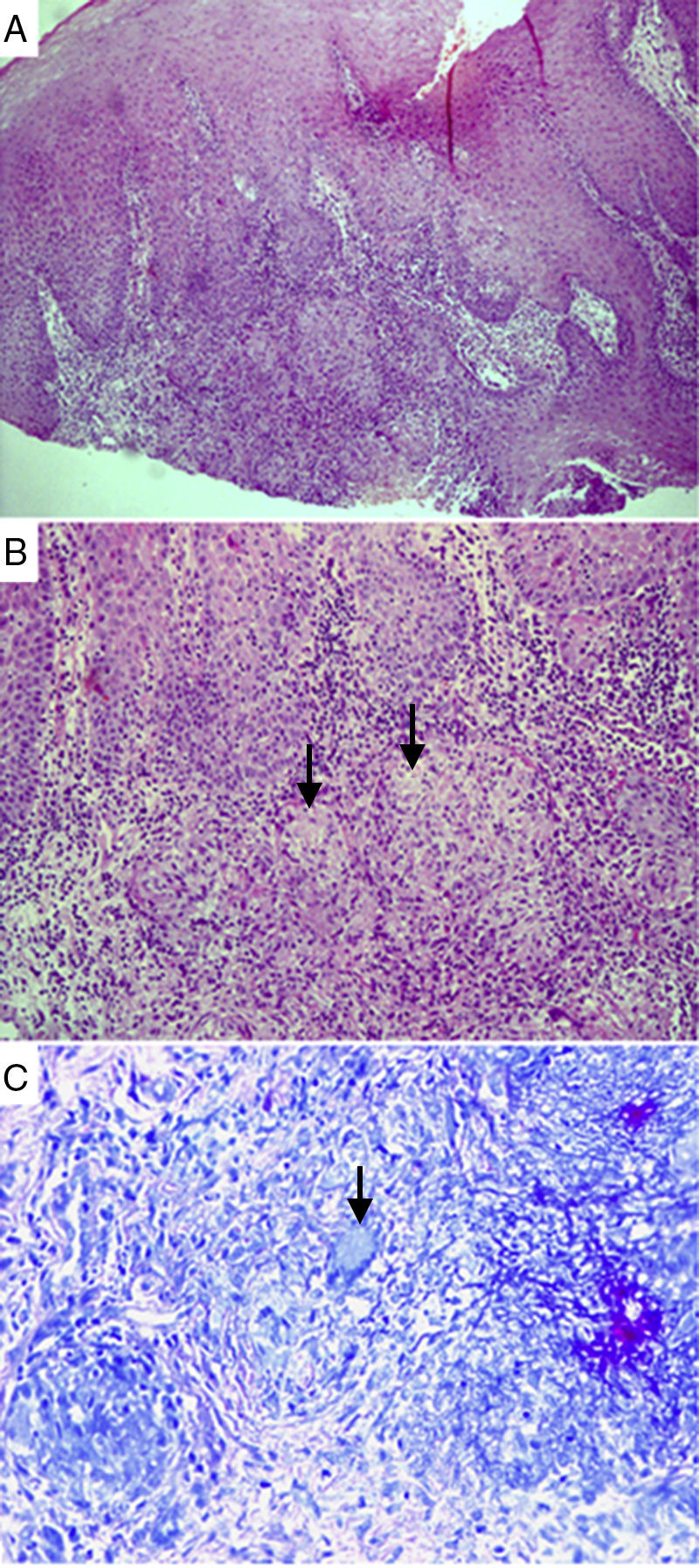
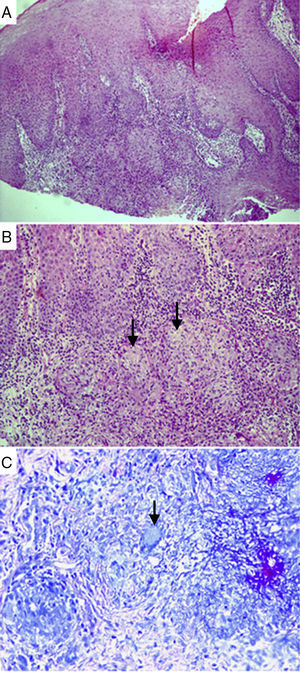
Histopathological analysis of a sample collected from oral TB lesions. Granulomas surrounded by intense mixed inflammatory infiltrate, with inflammatory cells inside the epithelium. Hematoxylin–eosin staining, 100× magnification (A). Well-shaped granulomas surrounded by epithelioid histiocytes and inflammatory cells. Arrows indicate incipient necrosis. Hematoxylin–eosin staining, 200× magnification (B). Arrow indicates giant Langhans cell, with nuclei distributed across the peripheral cytoplasm, in a necklace pattern. Langhans cells are typical of TB. Ziehl–Neelsen staining, 600× magnification (C).
Clinically, a patient infected with M. tuberculosis and presenting an active manifestation of TB may also exhibit signs and symptoms such as persistent and productive cough, night sweats, weight loss, and low morning fever. TB is an essentially airborne disease whose transmission depends on prolonged contact with an infected patient.4 The efficiency of transmission is a function of the patient's contagious potential (which is associated with M. tuberculosis load), the intensity and frequency of cough, and presence of lung cavitation (based on radiographic examination). In addition, the intensity and duration of contacts with a TB patient also are important to bring about the possibility of TB diagnosis.4 In the present case-report, the patient described the symptoms intermittently along different appointments, which made an early diagnosis of TB more difficult.
The tuberculine sensitivity assay, also called Mantoux test, is the standard procedure to diagnose TB. The assay includes the intradermal inoculation of a purified protein derivative of M. tuberculosis to assess the cellular immune response to the antigens. An inflammatory reaction takes place in M. tuberculosis sensitized patients. Inspection is conducted after 48–72h, and is valid for 7 days. The evaluation is based on the diameter of the inflammation area measured transversally against the longitudinal direction of the challenged forearm. An inflammation area over 10mm in immunocompetent subjects is considered a positive result. In immunocompromised patients, an area larger than 5mm indicate TB. In turn, the minimum size of inflammatory area in low-risk individuals and children under 15 years of age is 15mm. Although the Mantoux reaction is the method of choice in TB diagnosis, the test has a few limitations, such as the low sensitivity in immunocompromised patients (which points to the risk of false negative results), the difficulty to use in children, the subjective character of interpretations, and the need for a second appointment for confirmation purposes in some cases.4
Clinically, TB has several clinical forms. However, due to the low prevalence, the lesions characteristic of oral TB are often overlooked in the differential diagnosis of other oral lesions. It is assumed that oral TB lesions account for 0.1% to 5% of the infections caused by M. tuberculosis.5 Oral lesions caused by TB may be primary, which are rare and occur as a result of the direct inoculation of oral tissues, or secondary, due to hematogenous or lymphatic dissemination and extensions of nearby structures.1,6 Autoinoculation may take place upon direct interaction of infected mucus with a wound on the oral mucosa. Secondary oral TB is considered most prevalent in elderly patients, while the primary manifestation of the disease is more common in young individuals.7
Although the oral presentation of TB can be primary, in this case hematogenous spread is evident the hematogenous spread. However, since the pathological analysis revealed no acid fast bacilli a Mantoux assay was requested in addition to bronchoalveolar lavage in order to confirm the TB suspicion. If oral TB is diagnosed, it is important to attempt to locate a primary site of the disease before the former can be considered primary. This is important in order to assess the extent of disease activity as well as to monitor complications in involved organs. The quantity of the bacilli observed indicates the demonstration of the severity of disease on site.
Systemic and local factors also play an important role in the development of oral lesions. Examples of systemic factors are immunosuppression and the increase in virulence of pathogens.8 In turn, the list of local factors includes poor oral hygiene, local trauma, chronic inflammations, tooth eruption, surgical lesions, periodontal disease, caries, pulp exposure, cysts, and tooth abscesses.6,8 It is possible that the virulence of the M. tuberculosis strain also influences the involvement of oral structures.9 The patient herein described failed to comply with follow-up and treatment instructions for his chronic underlying diseases, thus worsening his TB and leading to the unfavorable outcome reported.
The oral manifestation of TB may present as an ulcerative, painless lesion on the palate, lips, or tongue, accompanied by persistent cervical lymphadenopathy.8 The differential diagnosis of TB ulcers includes a variety of ulcerative diseases and conditions, such as squamous cell carcinoma, trauma ulcers, aphthous stomatitis, syphilis ulcers, actinomycosis, Wegener's granulomatosis, sarcoidosis, leishmaniosis, zygomycosis, and Hansen's disease.9 It is important to highlight that oral ulcers may present a similar picture,10 requiring a diagnosis based on microscopic findings in addition to the Mantoux test and baciloscopy.11 The appropriate identification of oral TB is important not only for the patient, but also for the dentistry professionals and the community at large, since the patient is a potential source of transmission. Lesions in the head and neck region should always be considered in the differential diagnosis of TB, especially in high-risk populations. In case more than one clinical condition is suspected, a comprehensive laboratory investigation and thorax radiographic examination should be implemented to identify and control TB.9,12,13 Although oral manifestation of the disease is rare, a careful differential diagnosis of oral lesions is of paramount importance for a correct diagnosis, especially when there is suspicion of TB.14
In conclusion, despite being a rare manifestation of TB oral lesions should be included in the differential diagnosis of oral lesions in general, irrespective of the existence of pulmonary signs and symptoms, and whether the patient lives in a TB endemic region or not. Early and accurate diagnosis is essential in the establishment of appropriate treatment aiming at curing the patient with TB.
Conflicts of interestThe authors declare no conflicts of interest.