The timing of highly active antiretroviral therapy (HAART) after a tuberculosis diagnosis in HIV-infected patients can affect clinical outcomes and survival. We compared survival after tuberculosis diagnosis in HIV-infected adults who initiated HAART and tuberculosis therapy simultaneously to those who delayed the start of HAART for at least two months.
MethodsThe THRio cohort includes 17,983 patients receiving HIV care in 29 public clinics in Rio de Janeiro, Brazil. HAART-naïve patients at the time of a new TB diagnosis between September 2003 and June 2008 were included. Survival was measured in days from diagnosis of TB. We compared survival among patients who initiated HAART within 60 days of TB treatment (simultaneous – ST) to those who started HAART >60 days of TB treatment or never started (deferred – DT). Kaplan–Meier plots and Cox proportional hazards regression analyses were conducted.
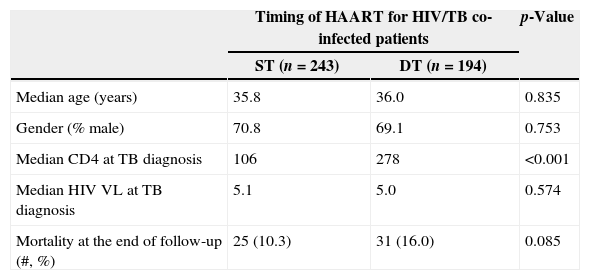
ResultsOf 947 patients diagnosed with TB, 572 (60%) were HAART naïve at the time of TB diagnosis; 135 were excluded because of missing CD4 count results. Among the remaining 437 TB patients, 56 (13%) died during follow-up: 25 (10%) among ST patients and 31 (16%) in DT group (p=0.08). ST patients had lower median CD4 counts at TB diagnosis than DT patients (106 vs. 278, p<0.001). Cox proportional hazards utilizing propensity score analysis showed that DT patients were more likely to die (adjusted HR=1.89; 95% CI: 1.05–3.40; p=0.03).
ConclusionHAART administered simultaneously with TB therapy was associated with improved survival after TB diagnosis. HAART should be given to patients with HIV-related TB as soon as clinically feasible.
Highly active antiretroviral therapy (HAART) significantly improves survival in HIV-infected patients with opportunistic infections, but the optimal time to initiate HAART in patients with HIV-related tuberculosis (TB) poses a challenge to clinicians and patients.1,2 Recent World Health Organization recommendations state that HIV-infected patients with active TB should initiate HAART as soon as possible after TB treatment, irrespective of CD4 cell count,3 though the quality of the evidence supporting these recommendations is characterized as low or moderate. The SAPiT trial in South Africa reported a 56% mortality reduction among patients starting HAART within four weeks after the start of TB therapy or within four weeks of completion of the intensive phase of TB therapy compared to those initiating HAART within four weeks of TB treatment completion.4 Further analysis of the SAPiT trial suggests that the protection conferred is equally strong if treatment is started at either time during TB treatment.5 The recent STRIDE trial reported no mortality difference between those starting HAART within two weeks of TB treatment initiation and those starting within 8–12 weeks after TB treatment start,6 though both trials report a significant drop in mortality among early starters with advanced immunosuppression. The CAMELIA clinical trial in Cambodia found a reduction of 34% among patients starting HAART eight weeks after TB treatment start compared to those starting HAART within two weeks in a population with 72% having a CD4<50cells/mm3.7 Studies in developed countries have shown that patients with very advanced HIV disease have better survival if HAART is given during TB treatment,8–11 though the risks of immune reconstitution inflammatory syndrome (IRIS),12,13 drug interactions and additive drug toxicity raise concerns about early initiation of HAART in TB/HIV co-infection.14 The three clinical trials suggest that HAART be initiated very early for those with advanced immunosuppression; however, those with higher CD4 counts at the time of TB diagnosis can wait to initiate HAART and not compromise survival, while also reducing the risk of IRIS and other complications.
Rio de Janeiro City, Brazil, has a large population of patients with TB/HIV co-infection, and TB is an important cause of death in individuals with AIDS.15 The TB/HIV in Rio (THRio) study was a cluster randomized trial16 designed to evaluate the impact of TB screening and isoniazid preventive therapy in HIV-patients receiving care at 29 public health units in Rio de Janeiro. In Brazil, HIV-infected patients have free access to HAART and TB therapy in public health clinics, and treatment in the private sector is not available. We conducted a survival analysis after TB diagnosis according to the timing of HAART initiation in the THRio cohort.
MethodsThis is an observational cohort survival analysis after the diagnosis of TB in HIV-infected patients receiving care in THRio clinics. We compared survival in patients receiving simultaneous HAART and TB therapy (HAART started within 60 days of TB diagnosis) with deferred HAART (HAART started >60 days but <365 days after TB diagnosis, or no HAART). Patients who started HAART >365 days after their TB diagnosis were censored at that point. Data collection for THRio has been described previously.16 In brief, 29 pubic primary health clinics treating HIV-infected patients were randomly assigned to begin implementing TB screening and isoniazid preventive therapy (IPT) in September 2005. Patient data were abstracted from medical records starting at the date of HIV diagnosis and included age, gender, HIV diagnosis date, history of HAART and date of initiation, history of opportunistic infections, CD4 counts, tuberculin skin tests, IPT, and viral loads. For the current analysis, patients were included if they (1) were HIV-positive by serologic testing; (2) had a first diagnosis of active TB between September 1, 2003 and June 30, 2008; (3) were not receiving HAART for more than 60 days at the time of TB diagnosis; and (4) were diagnosed with HIV either prior to TB diagnosis or within 30 days after TB diagnosis. TB was defined according to Brazilian guidelines, and included symptoms, X-rays, sputum smears and cultures. TB treatment was given to all patients using the Brazilian regimen of three drugs daily for two months followed by two drugs for four months.17
The primary outcome was all-cause mortality. The primary exposure of interest was time elapsed since HAART initiation, which was divided into two categories: HAART initiated within 60 days of TB treatment start date (simultaneous therapy (ST)) and HAART initiated more than 60 days after TB start date or never started during the study period (deferred therapy (DT)). Other covariates that were assessed included CD4 count at TB diagnosis, gender, and age. Patients were censored either at the last clinic visit, or on December 31, 2008, or at HAART start date if HAART start date was more than one year after TB treatment start date which ever occurred first. Death reporting was abstracted from patient medical records or from the National Mortality System (SIM), after linkage with the THRio cohort, using an algorithm described elsewhere.18 Date of TB diagnosis was the date of initiation of TB treatment recorded in the clinic charts. HAART was defined as the use of two nucleoside reverse transcriptase inhibitors plus a non-nucleoside reverse transcriptase inhibitor or one or two protease inhibitors in combination. Date of HAART initiation was abstracted from clinic charts. Date of death was the date recorded in the death certificate, as entered in the SIM. HAART was initiated at the physicians’ discretion, guided by the Brazilian Recommendations to Antiretroviral Therapy for HIV-infected Adults and Adolescents in use at the time of the study.19
Descriptive statistics were performed using t-tests for continuous variables and Fisher's exact test for categorical variables. Survival curves were examined using the Kaplan–Meier method, and the log rank test was used to assess differences in survival functions by group.
Propensity scores were calculated as follows: a logistic regression model was run having DT/ST as the response variable and gender, age and CD4 counts as co-variables. Predicted probabilities from the model were used to construct quintiles, which were included in the model as a stratifying variable. Cox proportional hazards regression analyses were conducted and propensity scores for HAART initiation by 60 days post-TB diagnosis were used to stratify the baseline hazard function in order to minimize bias from treatment by indication.
The THRio study was approved by the Research Ethics Committee of the Municipal Secretary of Health and Civil Defense of the Municipality of Rio de Janeiro, Brazil, and the Johns Hopkins Medicine Institutional Review Board. The study received a waiver of informed consent, as no direct contact with patients was undertaken by study staff and all data were deidentified prior to analysis.
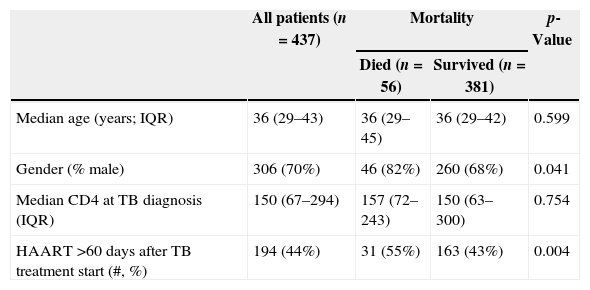
ResultsThere were 17,983 subjects in the THRio cohort for this analysis. Between September 2003 and June 2008, 947 cases of TB were diagnosed, of whom 572 had no history of exposure to HAART for greater than 60 days at TB diagnosis. One hundred thirty-five patients were excluded from the analysis because they did not have a CD4 count result available within four months of their TB diagnosis. Of the 437 TB/HIV patients in the current analysis, 78% of the cases were male, the median age was 36 years (IQ range: 29–43 years) and the median CD4 cell count at TB diagnosis was 150cells/mm3 (IQ range: 67–294 cells). Of the 389 (89%) who started HAART at or after TB treatment start date, 243 started within 60 days of TB treatment start date (ST), 125 started HAART >60 days and ≤365 days after TB treatment start date (DT), and 21 started HAART >365 days after TB treatment start date and were censored at HAART start date (DT). Forty-eight patients never started HAART in the study period (DT) and were censored as described. ST patients did not differ from DT patients in age, gender or RNA viral load at the time of TB diagnosis (Table 2). ST patients had lower median CD4 cell counts at the time of TB diagnosis (106 vs. 278cell/mm3, p<0.001). Fifty-six patients (13%) died during a median of 2.1 years of follow-up (2212 person-years), at a mortality rate of 7.6 deaths per 100 person-years. The median time to death was 338 days. Subjects who died had similar baseline CD4 counts and HIV viral loads at time of TB diagnosis as those who survived (Table 1).
Predictors of mortality in HIV/TB co-infected patients.
| All patients (n=437) | Mortality | p-Value | ||
|---|---|---|---|---|
| Died (n=56) | Survived (n=381) | |||
| Median age (years; IQR) | 36 (29–43) | 36 (29–45) | 36 (29–42) | 0.599 |
| Gender (% male) | 306 (70%) | 46 (82%) | 260 (68%) | 0.041 |
| Median CD4 at TB diagnosis (IQR) | 150 (67–294) | 157 (72–243) | 150 (63–300) | 0.754 |
| HAART >60 days after TB treatment start (#, %) | 194 (44%) | 31 (55%) | 163 (43%) | 0.004 |
Patient characteristics by timing of HAART for TB/HIV co-infection.
| Timing of HAART for HIV/TB co-infected patients | p-Value | ||
|---|---|---|---|
| ST (n=243) | DT (n=194) | ||
| Median age (years) | 35.8 | 36.0 | 0.835 |
| Gender (% male) | 70.8 | 69.1 | 0.753 |
| Median CD4 at TB diagnosis | 106 | 278 | <0.001 |
| Median HIV VL at TB diagnosis | 5.1 | 5.0 | 0.574 |
| Mortality at the end of follow-up (#, %) | 25 (10.3) | 31 (16.0) | 0.085 |
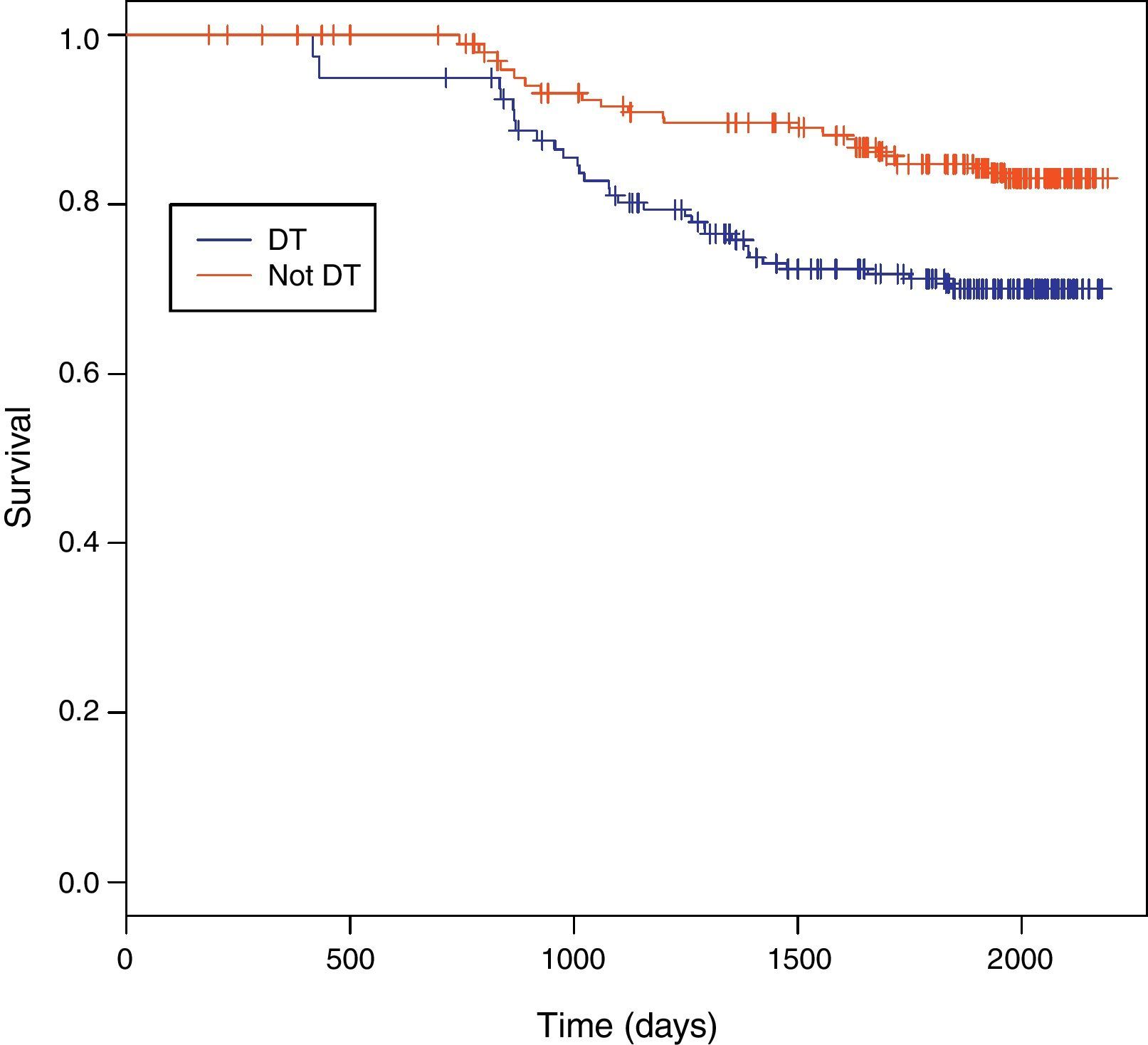
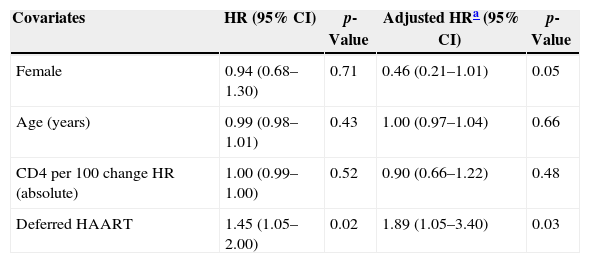
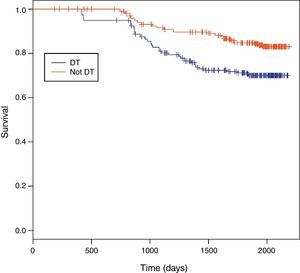
Among 243 TB patients in the ST group, 25 (10%) died during follow-up compared to 31 of 194 (16%) in the DT group (p=0.085). Kaplan–Meier estimates of survival after TB diagnosis stratified by timing of HAART initiation are shown in Fig. 1. ST patients were significantly less likely to die over the follow-up period than DT patients (log-rank test p=0.02). Univariate Cox analysis revealed that delaying therapy increased mortality risk 45% (HR=1.45; 95% CI: 1.05–2.00). Adjusted Cox proportional hazards modeling revealed an 89% increased risk in mortality among DT patients compared to ST patients (RH=1.89; 95% CI: 1.05–3.40; p=0.03), taking the propensity score into account (Table 3). No other covariates were associated with increased mortality.
Cox regression model, univariate and multivariate analysis.
| Covariates | HR (95% CI) | p-Value | Adjusted HRa (95% CI) | p-Value |
|---|---|---|---|---|
| Female | 0.94 (0.68–1.30) | 0.71 | 0.46 (0.21–1.01) | 0.05 |
| Age (years) | 0.99 (0.98–1.01) | 0.43 | 1.00 (0.97–1.04) | 0.66 |
| CD4 per 100 change HR (absolute) | 1.00 (0.99–1.00) | 0.52 | 0.90 (0.66–1.22) | 0.48 |
| Deferred HAART | 1.45 (1.05–2.00) | 0.02 | 1.89 (1.05–3.40) | 0.03 |
In this cohort of 437 patients with HIV-related TB who were HAART naïve at the time of TB diagnosis, not receiving HAART within 60 days after TB diagnosis was associated with an 89% increased risk of mortality. Patients who received HAART early had significantly lower CD4 cell counts at the time of TB diagnosis but had better overall survival. New WHO guidelines state that HAART should be initiated as soon as possible in all patients with HIV and active tuberculosis, regardless of CD4 cell count.3 Our data strongly support the use of HAART in all patients with HIV-related TB as early after TB treatment as possible in Brazil, with a reduction in mortality that was even greater than what has been reported in recent clinical trials.4–7
HAART has been shown to decrease the incidence of TB in Africa,20,21 Brazil,22,23 and other developed and developing countries,24–26 and HAART strongly reduces mortality among patients co-infected with HIV and TB10,11 as seen in our study. The protection offered by HAART has been consistently seen across CD4 cell strata, but most markedly in patients with most advanced immunodeficiency.26,4–7 In our study, CD4 at the time of TB diagnosis did not independently impact survival among co-infected patients, though CD4 was significantly lower in patients starting HAART early. Most likely, the increase in the CD4 cell count explains most of the protection afforded by HAART, although other factors, such as HIV viral load and levels of other T cell subpopulations, may play a role.26 Patients who fail to increase their CD4 counts after HAART initiation are at higher risk of recurrent TB.27
Survival between the ST and DT groups did not begin to diverge until about 1.5 years following TB diagnosis, well after TB treatment had been completed. The SAPiT Trial in South Africa and the CAMELIA Trial also did not see a significant survival benefit until after six months of TB therapy.4,7
This clinical cohort study has some limitations. Our data were collected from patient medical records, and information on drug toxicity and IRIS was generally not available. Drug-related toxicity and IRIS events occur more commonly at lower CD4 counts and may affect morbidity, though clinical trials show no impact of IRIS on mortality. In addition, the THRio study is based on data abstracted from medical charts, and exposure to HAART was allocated at physicians’ discretion, based on the Brazilian Recommendations for Antiretroviral Therapy for Adults and Adolescents,19 which guides HIV care in Brazil. These guidelines have recently been updated stating that for patients with pulmonary TB with a cavitary lesion, a CD4 cell count should be conducted 30 days after starting TB treatment28 and that HAART should be started if CD4 count is less than 350cells/mm3.
ConclusionWe showed that HAART initiated early after TB treatment in co-infected patients was associated with an 89% reduction in the risk of death compared to delayed HAART initiation. Thus, we provide further evidence of the importance of early HAART in increasing survival among HIV/TB co-infected patients. Strategies to promote the early diagnosis of HIV infection among TB patients and early initiation of HAART play important roles in improving the quality of care for patients co-infected with TB and HIV.
Conflicts of interestThe authors declare no conflicts of interest.
We thank the THRio participants and the THRio team, without whom this project would not have been possible.
Funding was provided by the Bill and Melinda Gates Foundation, grant 19790.01 to the Consortium to Response Effectively to the AIDS-Tuberculosis Epidemic (CREATE).