Tuberculosis is a leading cause of death among people living with human immunodeficiency virus. In sub-Saharan Africa, tuberculosis accounts for more than 78% of all deaths among people with human immunodeficiency virus.
ObjectivesTo assess tuberculosis treatment outcome and the associated factors in adult tuberculosis/human immunodeficiency virus co-infected patients in four public hospitals of eastern and southern zone of Tigray region, Ethiopia.
MethodologyInstitution based cross-sectional study design was used to examine secondary data from tuberculosis/human immunodeficiency virus co-infected patients attending four public hospitals of eastern and southern zone of Tigray, from January 2009 to August 2011. Systematic random sampling technique was used to select individual patient cards from the respective hospitals. Univariate analysis and multivariate logistic regression modeling was used to assess the impact of each variable in predicting treatment outcome.
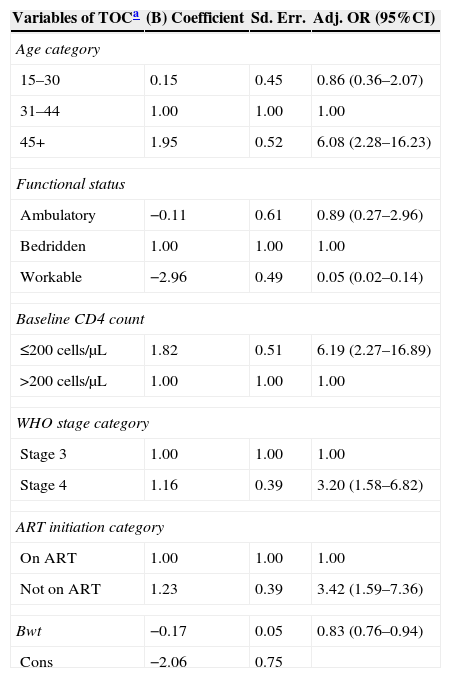
ResultsOut of 342 patients included, 199 (58.2%) patients completed treatment, 43 (12.6%) patients were cured, 88 (25.7%) died, 7 (2%) defaulted, and 5 (1.5%) patients failed treatment. Treatment success rate was around 71%. In the multivariate logistic regression analysis the factors that were strongly associated with unfavorable tuberculosis treatment outcomes were WHO stage IV (AOR=3.2, CI=1.58–6.82, p-value=0.001), age greater than 45 years (AOR=6.08, CI=2.28–16.23) and baseline CD4 count less than 200cells/μL (AOR=6.19, CI=2.28–16.89, p-value=0.001).
ConclusionThe rate of treatment success in this study was lower than the rate newly recommended by WHO. Therefore, efforts should be undertaken to improve treatment success rates of both diseases.
Globally it is estimated that almost 33% of all people living with HIV are co infected with TB and among these one in four deaths is due to TB. Where as in sub-Saharan Africa the co infection reaches for more than 70%. People with HIV are six times more likely to die during TB treatment. In the year 2009, approximately 400,000 people died of HIV-associated TB. In sub-Saharan Africa, it accounts for more than 78% of HIV-related TB deaths.1
According to the 2008 World Health Organization (WHO) report, the HIV epidemic has completely destabilized TB control in regions with high rates of HIV. For example, in one community outside of Cape Town, South Africa, TB patient case load increased six-fold between 1996 and 2004. The rates of TB in this community is over 150-fold higher than the national rates in many high-income countries, and this has been replicated right across southern Africa.2
Ethiopia is one of the highly affected countries by TB/HIV co-epidemic and ranked 7th among the 22 high TB burden countries in the world. The reported prevalence of TB/HIV co-infection has varied according to different authors and study site. However, the 2008 WHO Global Report estimates that in Ethiopia 40% of TB patients tested for HIV turned out positive.3
TB and HIV co-infection are associated with special diagnostic and therapeutic challenges and constitute an immense burden on healthcare systems of heavily infected countries like Ethiopia.3 TB/HIV co-infected patients have lower quality of life in all domains as compared to HIV infected patients without active TB.4
Despite the availability of effective drugs for treating both HIV/AIDS and TB, the co-management of TB and AIDS has proved very difficult largely because of non-adherence due to high pill burden, drug-drug interaction and side effects. There is also a worrisome convergence of multi-drug resistant or extensively drug-resistant tuberculosis in the setting of HIV infection causing a high mortality rate. To overcome this problem the WHO recommends that Highly Active Anti-retroviral therapy (HAART) should be given as early as possible within eight weeks of TB treatment initiation regardless of CD4 count.5
There is also evidence that early initiation of ART in people living with HIV significantly improves survival of co-infected patients and reduces excess risk for opportunistic disease or death, besides having a significant role in TB prevention.6,7
Studies from different countries have documented the treatment success of anti-TB regimen ranging from 30%8 to 83%.9 The treatment outcomes have significant association with socio-demographic attributes8,10 like age, sex, and marital status, in addition to clinical variables like TB category, HIV/AIDS co-infection and late ART initiation.11–13
So far, there is limited information available about predictors of treatment outcomes of TB patients co-infected with HIV in Ethiopia. Therefore, the aim of this study was to assess TB treatment outcomes categorized as favorable or unfavorable outcomes among HIV infected patients receiving anti-TB treatment and to identify the associated predictors for these treatment outcomes.
MethodologyStudy area and populationTigray is the northern region of Ethiopia covering an area of 54,569.25km2. In 2007, the population of the region was 4.3 million out of which 81% are living in rural area. Currently, a total of 14 public hospitals (one Referral, six Zonal and the remaining are District hospitals), 209 health centers and 538 health posts are available in Tigray. All of the hospitals are providing comprehensive service for patients with TB/HIV co-infection.
This study was conducted in four public hospitals located in Eastern and Southern zones of the region: Adigrat, Wukuro, Lemlem Karl and Alemata hospital. The study population was all TB/HIV co-infected adult patients registered in one of the above four hospitals and on TB treatment from January 2009 to August 2011.
Study designInstitutional based cross-sectional study design was used to examine TB treatment outcomes of HIV co-infected patients attending the afore-mentioned public institutions.
PopulationPatients aged ≥15 years, TB-HIV co-infected who were treated for at least TB, having complete record of treatment outcomes were included in the study. Treatment outcomes are categorized as favorable (cured, treatment complete) or unfavorable (died, defaulted or failed).
Sample size and sampling methodsSample size was determined using a single population proportion formula with 95% confidence interval and 5% margin of error, assuming 50% favorable treatment outcomes among TB/HIV subjects. Since the population was less than 10,000 the sample was inflated by a correction factor and ended up in 342 units. Then, the calculated sample size was distributed based on population proportion to size. All TB/HIV infected patients who registered for anti-TB treatment during January 2009–August 2011 were used as a sampling frame for selection. Systematic random sampling method was used to select individual patient cards from the respective hospitals.
Data collection and managementData were collected using prepared data extraction format (checklist). The format was developed by considering variables to be studied which were found in the registration book, follow-up card of the patients, and baseline studies. It was pre-tested and updated. Data collectors and one TB clinic head nurse/health officer from each hospital received a one-day training that emphasized exclusion of incomplete cards, inclusion and exclusion criteria, and recording the exact information from the follow-up card.
Patient records were identified from the database or TB treatment follow-up medical record cards. One facilitator from each hospital card room was used to identify individual cards. The recorded (collected) data were checked for completeness by the principal investigators for its completeness on a daily basis.
Data analysisData were entered, cleaned and analyzed using SPSS version 16. Descriptive statistics aimed to summarize patients’ characteristics across different categories of the independent variables. The effect of categorical and continuous predictor variables on the outcome of interest were checked using normality test of the distribution of values of the continuous variables, collinearity statistics and interaction between the significant variables.
Multicollinearity among predictor variables was assessed using the variance inflation factor (VIF) and tolerance (1/VIF). Those variables with greater than 10 VIF or less than 0.1 tolerance values were considered as having no collinearity. VIF Model adequacy was checked and assessed using −2 log likelihood, Cox & Snell R Square, Nagelkerke R Square, and goodness-of-fit.
To assess the relation of each predictor variable and the treatment outcome, univariate logistic regression model was used. Those variables with a p-value of less than 0.05 were included in the multivariate logistic regression model by backward stepwise entry method for assessing predictive power of those variables. A chi-square test was also performed to assess the association between the dependent and individual independent variables and combination of them to identify the significance of predictor variables.
Ethical considerationsEthical clearance was obtained from the Institutional Review Board (IRB) of the College of Health sciences, MU. Supporting letters were obtained from Tigray Health Bureau to the selected study Hospitals. Hospital Managers were contacted and permission was secured. Name and other identifiers of patients were not recorded on the questionnaires. The filled documents were archived properly to ensure confidentiality and no third person had access to the data.
ResultSocio-demographic characteristicsA total of 342 HIV/TB co-infected patients under TB treatment were included; 186 (54.4%) males, mean age 34 years (95% CI=33.01–35.12; SD=9.87), being 35 years (95% CI=33.7–36.9, SD=10.2) for males and 33 years (95% CI=31.5–34.3, SD=9.4) for females. Most of them (83.6%) were in the productive age group (15–44 yrs). The mean baseline weight was 44.8kg (95% CI=44.02–45.5, SD=6.9). Three hundred and eight (90.1%) participants were Orthodox followers, 181 (52.9%) were married; 209 (61.1%) study subjects attended primary school and 98 (28.6%) were employed.
Analysis of the factorsThe factors with a p-value less than 0.05 were included in multivariate logistic regression analysis and if the 95% CI of the odds ratio excluded one. Not to be on ART had 3.42 times higher risk of having unfavorable treatment outcome than those patients on ART before starting TB treatment (Adj. OR=3.42, 95% CI=1.59–7.36, p-value=0.002; Table 1).
Association of independent factors (predictive variables) including ART category, WHO HIV/AIDS stage, baseline CD4 count, and socio-demographic attributes with the treatment success (dependent variable).
| Variables of TOCa | (B) Coefficient | Sd. Err. | Adj. OR (95%CI) |
|---|---|---|---|
| Age category | |||
| 15–30 | 0.15 | 0.45 | 0.86 (0.36–2.07) |
| 31–44 | 1.00 | 1.00 | 1.00 |
| 45+ | 1.95 | 0.52 | 6.08 (2.28–16.23) |
| Functional status | |||
| Ambulatory | −0.11 | 0.61 | 0.89 (0.27–2.96) |
| Bedridden | 1.00 | 1.00 | 1.00 |
| Workable | −2.96 | 0.49 | 0.05 (0.02–0.14) |
| Baseline CD4 count | |||
| ≤200cells/μL | 1.82 | 0.51 | 6.19 (2.27–16.89) |
| >200cells/μL | 1.00 | 1.00 | 1.00 |
| WHO stage category | |||
| Stage 3 | 1.00 | 1.00 | 1.00 |
| Stage 4 | 1.16 | 0.39 | 3.20 (1.58–6.82) |
| ART initiation category | |||
| On ART | 1.00 | 1.00 | 1.00 |
| Not on ART | 1.23 | 0.39 | 3.42 (1.59–7.36) |
| Bwt | −0.17 | 0.05 | 0.83 (0.76–0.94) |
| Cons | −2.06 | 0.75 | |
All of the public hospitals and most of the public health centers of Tigray had implemented the TB/HIV integrated activities to tackle co-infection in that region of the country. Implementation of these activities also addresses areas of mutual interest for control programs of the two diseases and ultimately contributes immensely to address this dual epidemic that has had a tremendously negative impact on TB treatment outcomes.
The rate of treatment success (cured or complete) was lower (71%) than the 75% to 85% observed in other studies conducted in Districts of southern India, teaching hospitals in southern India, South Africa in 2009 during the ART rollout, as well as the target rate aimed by the national TB and Leprosy control program of Ethiopia.9,11,12,14 Nonetheless, our rate was higher than the 29.5%–66.3% found in other studies conducted in Gonder teaching hospital, north west Ethiopia, and university college hospital, Ibadan, Nigeria.8,15|
The death rate (25.7%) of the study group was higher when compared to other studies conducted in teaching hospital of southern India, south Africa during the ART rollout and Gonder teaching hospital.8,9,12 On the contrary, it was lower than the 29.95% to 30% found in other studies conducted in Addis Ababa public health centers and districts of southern India.10,11 However, it was similar to death rate (25.7%) encountered in the study conducted in Gombe state, Nigeria.16 This implies that it needs further reconsideration of the guidelines and the collaborative activity from sectors and other stakeholders to improve the favorable treatment outcome.
The default rate (2%) seen in the study was lower compared to rates (8.2%–18.3%) found in other studies conducted in Gonder teaching hospital, Ethiopia and teaching hospitals of south India.8,9 The treatment failure rate (1.5%) of the present study was lower than the rates observed in other study conducted at the university college hospital, Ibadan, Nigeria (10.6)15 and at the teaching hospital south India (2.2%).9 It was higher than the rate found in Gonder teaching hospital Northwest Ethiopia (0.2%).8
The rates across the studied hospitals Adigrat, Alamata, Wukuro, and Lemlem Karl were respectively 8.8%, 12.7%, 16.7%, and 17.5% for cure; 52.8%, 57.6%, 59.5%, and 70.2% for completeness; 1.6%, 3.4%, 2.4%, and 0% for defaulting; 35.2%, 25.4%, 16.7%, and 12.3% for death; and 1.6%, 0.8%, 4.8%, and 0% for treatment failure. The results shown in Lemlem Karl hospital were better when compared to the other three hospitals and this finding warrants further investigation.
The overall treatment success rates were 87.8%, 76.3%, 70.3%, and 61.6%, respectively, in Lemlem Karl hospital, Wukuro hospital, Alamata hospital, and Adigrat hospital. This suggests that the treatment success rate varies among hospitals. This may be due to difference in human resource quality and quantity, and availability of diagnostic facilities. The factors associated with the observed difference warrant further investigation.
Even though it was not significant in this study, the importance of chemoprophylaxis, such as CPT, to prevent other OIs may also play a significant role in avoiding deaths particularly when access to ART is limited, as in developing countries.11 Though WHO and Ethiopian National guidelines recommend that all HIV-infected patients diagnosed with TB should receive CPT, 26% of the patients had not received CPT at time of initiation of anti-TB treatment in the descriptive study.
In the multivariate logistic regression analysis, patient's age, ART initiation, baseline CD4 category, WHO stage, functional status, and baseline weight were significantly associated with unfavorable TB treatment outcome. Age category was independently associated with unfavorable TB treatment outcome in this study. Patients with age ≥45 years had significantly higher probability of death compared to patients aged <45 years (AOR=6.08, 95% CI=2.28–16.23). This was consistent to other studies from developing countries. A study conducted in South Africa identified that being aged ≥41 years significantly increased the risk of unfavorable treatment outcome of TB/HIV co-infected patients (RR=3.2, 95% CI=2.4–7.7).12 Thus, clinicians working in TB/HIV clinics should give closer attention to elderly patients to enhance the desired outcomes of the TB/HIV collaborative activities.
No initiation of ART was found to be an independent risk factor for unfavorable TB treatment outcomes. Those patients who were not on ART had 3.42-fold increase in risk of unfavorable outcomes (AOR=3.42, 95% CI=1.59–7.36). Similarly, a study conducted in South India showed that not to be on ART had 4.9-fold increase in risk for unfavorable treatment outcome (AOR=4.90, CI=1.85–12.96).11 There is enough evidence showing that patients with TB and HIV who did not receive ART had a significantly higher risk of unfavorable treatment outcome, particularly death.11,12,17
Despite the apparent benefit associated with ART, only 48% (164) of the HIV-infected TB patients received ART in our study. Similarly, most HIV-infected TB patients in many developing countries still cannot access ART primarily due to economic barriers and limited coverage.11 So these findings highlight the importance of expanding and improving delivery of ART services as a priority and reconsideration of the program guidelines for ART initiation in HIV-infected TB patients.
In this study, baseline body weight was significantly associated with higher unfavorable treatment outcome. A 1kg increase in baseline weight was associated with 17% decrease in the risk of unfavorable outcome (AOR=0.83, 95% CI, 0.77–0.94). Similar finding was observed in South India, in which body weight at initiation of anti-TB treatment was a significant risk factor of death during anti-tuberculosis treatment period (19).17 Therefore, it might be important to reinforce the existing DOTS program to include nutritional support for underweight patients in order to achieve better treatment outcome.
ConclusionThere was high rate of death (25.7%) or overall unfavorable treatment outcome (death, defaulting and failure=29.2%) among TB/HIV co-infected. This is a serious public health concern that needs to be addressed. The independent risk factors for unfavorable treatment outcome found in this study were non-initiation of ART before the initiation of anti-TB treatment, having low baseline weight, being older, having low baseline CD4 count (less than 200cells/μL), to be at WHO stage IV, and having bedridden or ambulatory functional status.
Based on these findings we recommend that governmental and non-governmental organizations, local managers and other stakeholders involved in TB/HIV management should facilitate for implementation and enforce early application of the new WHO Guidelines that recommend that any patient with CD4 count less than 350cells/μL should start ART to improve the treatment outcome of TB/HIV co-infected patients.
There should be support and careful follow-up for older patients. There should be early detection and treatment of opportunistic infection, especially TB for those in WHO stage IV, e.g. strengthening nutritional support like plump net to those with low baseline weight at the initiation of TB treatment. In addition, early detection of TB cases in HIV-infected patients should be strengthened in order to improve treatment outcome.
Limitation of the studyThe major limitation of the study was related to the use of secondary data. As different literature pointed out, there are factors assumed to impact on treatment outcome of TB/HIV co-infected patients, which may not be available in the secondary data, such as alcohol consumption and cigarette smoking. There were also missing values of the predictor variables. However, we minimized the risk of incomplete data by having multiple source of information (registration and card) for independent and dependent variables and by limiting the objective to those important variables that could be collected from the sources. The study used all forms of TB due to lack of enough patients with smear-positive pulmonary TB. The exact tools used to diagnose other forms of TB were difficult to ascertain.
Conflicts of interestThe authors have no conflicts of interest to declare.