To describe the investigation of latent tuberculosis infection and indication for isoniazid preventive therapy in children and adolescents evaluated at the children's hospital.
MethodsThis retrospective study examines all latent tuberculosis infection subjects with indication for isoniazid preventive therapy attended during 2002–2009 at the pulmonology outpatient clinic from children's hospital in Rio de Janeiro, Brazil. The subjects were classified into three groups by origin: (G1) primary and secondary health units; (G2) children's hospital-pulmonology outpatient clinic; and (G3) children's hospital-specialty outpatient clinics. The association between the variables examined and G1 was analyzed using univariate analysis.
ResultsOf the 286 latent tuberculosis infection cases included 169 (59.1%) were from G1, 56 (19.6%) from G2, and 61 (21.3%) from G3. Latent tuberculosis infection diagnosis without isoniazid preventive therapy prescription was present in 142 (49.6%) cases before arrival at the pulmonology outpatient clinic: 135 (95.1%) from G1, three (2.1%) from G2, and four (2.8%) from G3. Variables associated with G1 were presence of isoniazid preventive therapy criteria before attending the pulmonology outpatient clinic (OR: 62.3; 26.6–146.2), negative HIV infection status (OR: 9.44; 1.16–76.3); contact with pulmonary tuberculosis (OR: 5.57; 1.99–15.5), and residing in Rio de Janeiro city (OR: 1.89; 1.04–3.44).
ConclusionStrategies that increase latent tuberculosis infection identification and isoniazid preventive therapy prescription in primary and secondary health units are urgently needed.
Tuberculosis (TB) affects 8.8 million people every year.1 It is estimated that 10–15% of these cases occur in children and adolescents under 15 years of age. This percentage is most likely higher due to underreporting of TB in this age group.2
Latent TB infection (LTBI) is estimated to affect one third of the world population.1 Childhood LTBI usually arises from contact with adult pulmonary TB (PTB) patients.3
Approximately 5–10% of individuals with LTBI may progress to active TB throughout life, and this progression may reach 40% in younger children.4,5 Isoniazid preventive therapy (IPT) in this age group reduces the likelihood of disease progression to less than 0.5%.5 Worldwide, identifying contacts of adults with PTB and treating LTBI is considered a priority.1
In most cases, LTBI progresses almost imperceptibly from the clinical point of view and can be confused with viral or bacterial infections when symptomatic, especially among children and adolescents. Reactivity to the tuberculin skin test (TST) combined with exclusion of active TB6 is required for LTBI diagnosis.
Recent guidelines of the International Union Against Tuberculosis and Lung Disease (IUATLD) in partnership with other institutions encourage strategies for the care and training qualification of Primary Health Care (PHC) professionals, considered the individuals’ gateway to health care. Expanding the investigation of LTBI and active TB in children and directing asymptomatic child of PTB contacts to IPT at this health care level is recommended.7
However, in the literature, data on the effectiveness of such actions in the PHCs of countries with high TB burden, such as Brazil, are scarce.8–13
Operational analyses in developing countries showed that less than a quarter of the pediatric population eligible for IPT received it, showing a devaluation of this preventive measure for childhood TB, particularly by health care professionals.13
Durovni8 reported that only 18.4% of PTB contacts were examined in a shantytown in Rio de Janeiro/Brazil in 2011 where 100% of the community was visited by local health care workers, and no information was available regarding IPT implementation.
This study aims to describe the investigation of LTBI with subsequent IPT indication in children and adolescents attended in a reference hospital in Rio de Janeiro/Brazil, mostly coming from primary and secondary health units (HUs).
Study population and methodsThis is a descriptive, longitudinal and retrospective study conducted at the pulmonology outpatient clinic (POC) of the Jesus Municipal Hospital (Hospital Municipal Jesus – HMJ), located in the neighborhood of Vila Isabel, Rio de Janeiro/Brazil, from January 2002 to December 2009. HMJ is an exclusively pediatric HU, a municipal reference for the investigation and treatment of LTBI and TB either associated or not with the human immunodeficiency virus (HIV) in patients under 15 years of age. The POC receives children and adolescents from the pediatric department and from other specialty departments of the hospital or referred from external HUs.
This study was approved by the Ethics and Research Committee of the Clementino Fraga Filho University Hospital (Hospital Universitário Clementino Fraga Filho – HUCFF) under research protocol number 068/11 CAAE.
All children under 15 years of age with LTBI referred for IPT in the POC and living in the state of Rio de Janeiro/Brazil were included in the study, and no individuals were excluded. Data were obtained from the medical records of individuals who received IPT in the POC.
Considering the origin of individuals referred to the POC, the following study groups were formed:
- •
Group 1 (G1): referred from primary and secondary HUs.
- •
Group 2 (G2): referred from the HMJ general pediatric outpatient clinic.
- •
Group 3 (G3): referred from other HMJ pediatric specialty outpatient clinics.
The individuals studied were evaluated at two different times: at the first visit to the POC and at the follow-up, when the indication for IPT was defined. Active TB was excluded on clinical and epidemiological grounds, clinical examination, chest radiography, and, when indicated and feasible, gastric lavage fluid and/or induced sputum examination by Ziehl-Neelsen and culture for mycobacteria. The Brazilian scoring system recommended for childhood of PTB14 was applied to these individuals.
Socio-demographic, clinical, radiological, and laboratory variables were recorded in a form specifically designed for the study.
At the time of the study, IPT was indicated according to the guidelines of the Brazilian Ministry of Health (Ministério da Saúde – MS)14 for children and adolescents under 15 years of age with no signs compatible with active TB.
There was indication for repeating TST after 8–12 weeks in children and adolescents with initial TST<10mm. Individuals with tuberculin skin test conversion (TSTC), defined as an increase of at least 10mm compared to the previous TST, would also be referred for IPT after exclusion of active TB.14
The data collection form was developed using the software Access 1998, where the database was stored. The analyses were performed using the software Statistical Package for the Social Sciences version 17.0 for Windows. The variables studied were tested for association with the external origin of the individuals. The significance level was set at p<0.05. The chi-square test, or Fisher's exact test when indicated, were used to examine associations. Logistic regression techniques were not indicated, as some variables created duplication in statistical analysis.
ResultsIn total, 286 children and adolescents were included in the study. They had been referred to the POC with suspected active TB or LTBI due to being contacts of adults with PTB or not, symptomatic or not, and reactive or not to TST. All were diagnosed with LTBI and indicated for IPT at the POC.
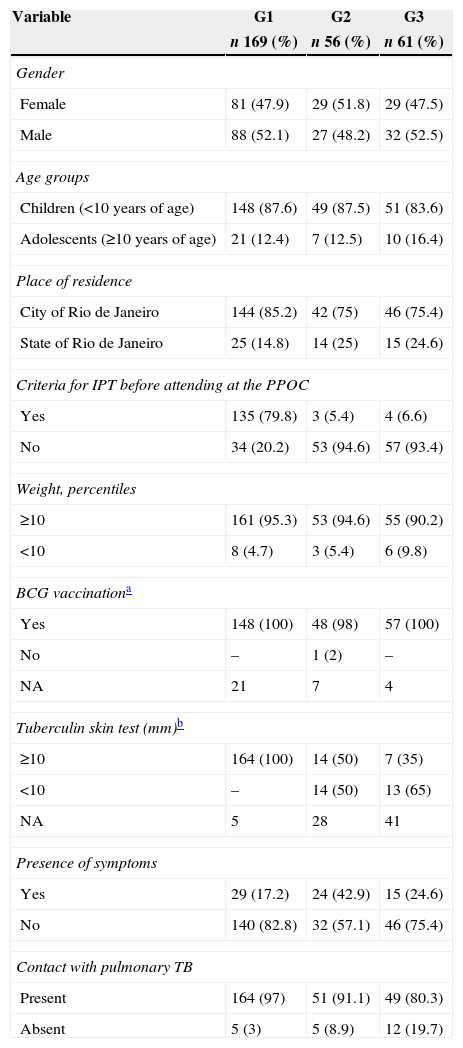
The individuals were distributed among the following groups: G1, 169 (59.1%); G2, 56 (19.6%); and G3, 61 (21.3%). Table 1 shows the clinical and epidemiological characteristics of each group at their first visit to the POC. G1 showed greater proportion of individuals with LTBI diagnosis referred to the POC with no prescription of IPT at the place of origin (135, 79.8%), from the city of Rio de Janeiro (144, 85.2%), with TST≥10mm (164; 100%), who reported contact with PTB (164; 97%), and who exhibited fewer symptoms (29; 17.1%).
Distribution of clinical and epidemiological characteristics according to study groups in 286 individuals at the first visit to the PPOC. HMJ-RJ, 2002–2009.
| Variable | G1 | G2 | G3 |
|---|---|---|---|
| n 169 (%) | n 56 (%) | n 61 (%) | |
| Gender | |||
| Female | 81 (47.9) | 29 (51.8) | 29 (47.5) |
| Male | 88 (52.1) | 27 (48.2) | 32 (52.5) |
| Age groups | |||
| Children (<10 years of age) | 148 (87.6) | 49 (87.5) | 51 (83.6) |
| Adolescents (≥10 years of age) | 21 (12.4) | 7 (12.5) | 10 (16.4) |
| Place of residence | |||
| City of Rio de Janeiro | 144 (85.2) | 42 (75) | 46 (75.4) |
| State of Rio de Janeiro | 25 (14.8) | 14 (25) | 15 (24.6) |
| Criteria for IPT before attending at the PPOC | |||
| Yes | 135 (79.8) | 3 (5.4) | 4 (6.6) |
| No | 34 (20.2) | 53 (94.6) | 57 (93.4) |
| Weight, percentiles | |||
| ≥10 | 161 (95.3) | 53 (94.6) | 55 (90.2) |
| <10 | 8 (4.7) | 3 (5.4) | 6 (9.8) |
| BCG vaccinationa | |||
| Yes | 148 (100) | 48 (98) | 57 (100) |
| No | – | 1 (2) | – |
| NA | 21 | 7 | 4 |
| Tuberculin skin test (mm)b | |||
| ≥10 | 164 (100) | 14 (50) | 7 (35) |
| <10 | – | 14 (50) | 13 (65) |
| NA | 5 | 28 | 41 |
| Presence of symptoms | |||
| Yes | 29 (17.2) | 24 (42.9) | 15 (24.6) |
| No | 140 (82.8) | 32 (57.1) | 46 (75.4) |
| Contact with pulmonary TB | |||
| Present | 164 (97) | 51 (91.1) | 49 (80.3) |
| Absent | 5 (3) | 5 (8.9) | 12 (19.7) |
G1, individuals referred from external primary and secondary HUs; G2, individuals referred from the HMJ general pediatric outpatient clinic; G3, individuals referred from other HMJ pediatric specialty outpatient clinics; HMJ, Jesus Municipal Hospital; NA, not available; PPOC, pediatric pulmonology outpatient clinic; TB, tuberculosis.
At the first visit to the POC, when investigating suspected cases of LTBI or active TB, a TST was requested for 74 individuals who were not tested before coming to the unit, aiming to complement the investigation. Among the 286 individuals analyzed, 272 (95%) were tested. Of these individuals, 212 (74.1%) had been tested before their first visit to HMJ, and 60 (21%) were tested after their first visit. Regarding the TST result, 78% of individuals had induration ≥10mm; 6%≥5–10mm; and 11%<5mm.
Reactivity to TST<10mm at the time of indication for IPT at the POC was seen in 49 (17%) patients. Of these patients, 26 (53%) underwent a new TST (within 6–8 weeks) to search for tuberculin contagiosum (TC), which occurred in 19/26 (73%) cases.
The 68 (24%) individuals who were symptomatic at the first visit were followed-up until complete resolution of symptoms, at which time IPT was indicated. Antimicrobial drugs were prescribed to 58 (85%) of them. The most frequent diagnoses were pneumonia in 31 (53%), and sinusitis in 15 (26%). All individuals underwent chest radiography. Abnormalities compatible with lower respiratory tract infections were found in 27 (39.7%) symptomatic individuals. These individuals exhibited further normal control radiological examination, some after appropriate antimicrobial treatment, at which time IPT was begun.
Among the 286 individuals studied, 18/230 (7.8%) already exhibited positive serology for HIV in their first visit to the POC. The presence of HIV was searched for in 56/268 individuals with unknown HIV serostatus, and all results were negative.
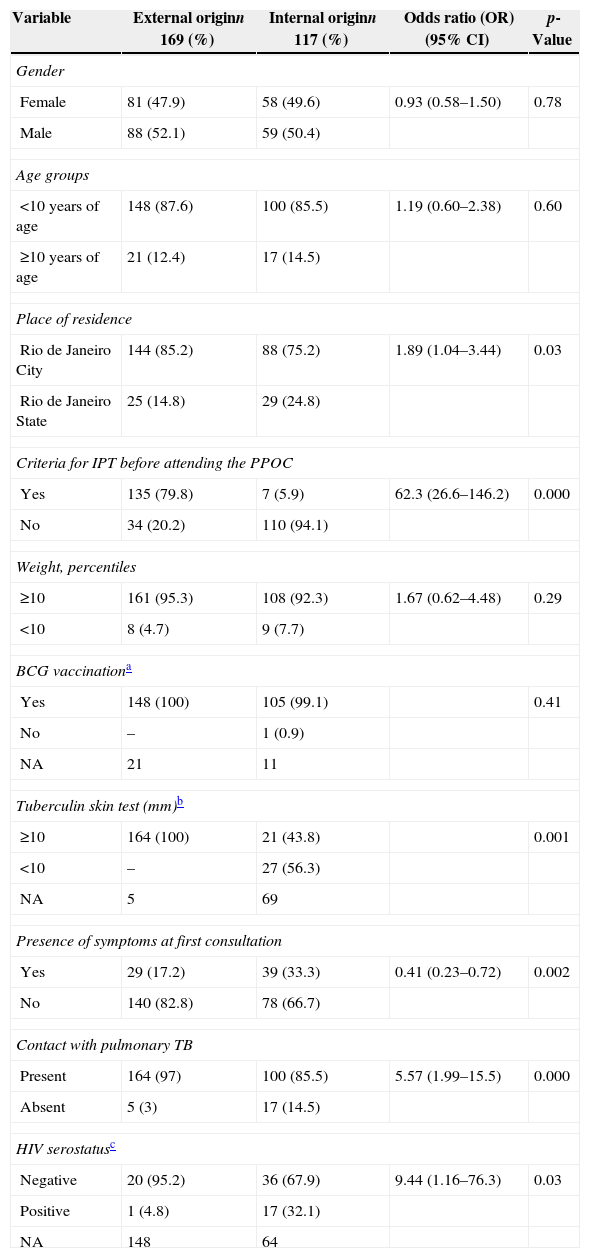
The comparative analysis between individuals with external (G1) and internal origin (G2 and G3), according to clinical and epidemiological characteristics, is described in Table 2.
Distribution of clinical and epidemiological characteristics according to internal and external origin of the 286 individuals referred to the PPOC. HMJ-RJ, 2002–2009.
| Variable | External originn 169 (%) | Internal originn 117 (%) | Odds ratio (OR)(95% CI) | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Female | 81 (47.9) | 58 (49.6) | 0.93 (0.58–1.50) | 0.78 |
| Male | 88 (52.1) | 59 (50.4) | ||
| Age groups | ||||
| <10 years of age | 148 (87.6) | 100 (85.5) | 1.19 (0.60–2.38) | 0.60 |
| ≥10 years of age | 21 (12.4) | 17 (14.5) | ||
| Place of residence | ||||
| Rio de Janeiro City | 144 (85.2) | 88 (75.2) | 1.89 (1.04–3.44) | 0.03 |
| Rio de Janeiro State | 25 (14.8) | 29 (24.8) | ||
| Criteria for IPT before attending the PPOC | ||||
| Yes | 135 (79.8) | 7 (5.9) | 62.3 (26.6–146.2) | 0.000 |
| No | 34 (20.2) | 110 (94.1) | ||
| Weight, percentiles | ||||
| ≥10 | 161 (95.3) | 108 (92.3) | 1.67 (0.62–4.48) | 0.29 |
| <10 | 8 (4.7) | 9 (7.7) | ||
| BCG vaccinationa | ||||
| Yes | 148 (100) | 105 (99.1) | 0.41 | |
| No | – | 1 (0.9) | ||
| NA | 21 | 11 | ||
| Tuberculin skin test (mm)b | ||||
| ≥10 | 164 (100) | 21 (43.8) | 0.001 | |
| <10 | – | 27 (56.3) | ||
| NA | 5 | 69 | ||
| Presence of symptoms at first consultation | ||||
| Yes | 29 (17.2) | 39 (33.3) | 0.41 (0.23–0.72) | 0.002 |
| No | 140 (82.8) | 78 (66.7) | ||
| Contact with pulmonary TB | ||||
| Present | 164 (97) | 100 (85.5) | 5.57 (1.99–15.5) | 0.000 |
| Absent | 5 (3) | 17 (14.5) | ||
| HIV serostatusc | ||||
| Negative | 20 (95.2) | 36 (67.9) | 9.44 (1.16–76.3) | 0.03 |
| Positive | 1 (4.8) | 17 (32.1) | ||
| NA | 148 | 64 | ||
HIV, human immunodeficiency virus; HMJ, Jesus Municipal Hospital; NA, not available; PPOC, pediatric pulmonology outpatient clinic; TB, tuberculosis.
In the univariate analysis, a significant association was observed between external origin (HUs) and the following factors: residence in the city of Rio de Janeiro (p=0.03); presence of LTB criteria with no IPT (p=0.000); positive TST (p=0.001); lower proportion of symptoms at first visit (p=0.002); history of contact with PTB (p=0.000); and HIV-negative serostatus (p=0.03).
DiscussionThis study analyzed individuals referred to the POC at CH with presumed TB/LTBI. At the first visit, approximately half of the cases had previously established criteria for LTBI diagnosis and indication for IPT (history of contact with PTB, TST≥10mm, absence of symptoms) according to the National Brazilian Guidelines.14 Additionally, 80% (135/169) of those referred from primary and secondary HUs (G1) presented with criteria for an LTBI diagnosis, and IPT was not indicated at that level.
In our study, there was a significant association between external origin (G1) and negative HIV infection status. We can speculate that HIV-infected patients received IPT at the primary and secondary HUs faster than patients not infected with HIV due to greater dissemination to health care professionals of guidelines for LTBI diagnosis in HIV-infected patients. It is noteworthy that almost all individuals infected with HIV were referred from other HMJ clinics to the POC for IPT prescription, even after active TB was ruled out, confirming the difficulty faced by the health care professionals, even in pediatric hospitals, in ruling out TB for subsequent IPT prescription.4,14
In Brazil, we found no information in the literature regarding the referral of children and adolescents from PHC units to reference units in relation to LTBI diagnosis and indication for IPT. Similar to the reports of Banu Rekha et al.15 and Tornee et al.16 in India and Thailand, respectively, we assume that in our study, the factors associated with no indication for IPT in the population that came from primary and secondary HUs may be related to the following: limited acceptance or ignorance of the importance of IPT by health care professionals, ignorance of standard diagnostic criteria for LTBI by prescribing physicians, and low effectiveness of measures for TB control in children and adolescents in these HUs. This low effectiveness could also be related to the fact that children have a history of being neglected regarding TB control because they contribute only minimally to spreading TB in the community.17
Moreover, we cannot rule out the scarcity of resources for performing TST and chest radiography in some primary and secondary HUs, which could explain the low resolution in pediatric cases of suspected active TB for ruling out TB and starting IPT.8,9 The lack of trained human resources in childhood TB in primary and secondary HUs in Rio de Janeiro could also be one of the reasons for difficulties in initiating IPT in this population, as most children and adolescents referred from those units to the POC had no symptoms that justified a thorough evaluation for active TB and difficulty in prescribing IPT.
In studies with Asian children, without specifying the health care level where they were conducted, doctors cited insufficient dissemination of guidelines for LTBI diagnosis, difficulties of the health system in implementing IPT, and fear of creating unnecessary adverse effects as reasons for deficient IPT prescription.15,16 In Malawi, the barriers cited were: lack of materials to perform TST; lack of equipment to perform chest radiography; lack of trained personnel to interpret the test results; and high workload of health care professionals under poor working conditions18. In Indonesia, the caregivers of child and adolescent PTB contacts who underwent screening for active TB faced obstacles to the screening process because they encountered difficulties in arriving at the HU due to transportation expenses and lost workdays. This way, 92% of the eligible child and adolescent contacts for LTBI/active TB were lost after initial evaluation.19
In a study in Salvador state, Brazil, Monroe et al.20 also found that primary and secondary health care professionals were insufficiently trained for TB control, overloaded with work, and minimally supervised, resulting in noncompliance with National Brazilian Guidelines for managing the disease. In the Rocinha shantytown of Rio de Janeiro in 2011, which had high primary health coverage, Durovni8 reported that after 100% of the community was visited by local health professionals, only 18.4% of close contacts were screened for LTBI. Recently, in another study conducted in the same shantytown of Rio de Janeiro, Machado21 reported the difficulty in tracking child contacts of patients with PT (children's daily contact network is not limited to the residents of a household, and health care professionals cannot ensure security in working with children). The authors also report that family physicians (who are not specialists and are focused on the adult population) exhibited difficulties in dealing with children and considered them the responsibility of the pediatric specialist. Conversely, in an operational study conducted in Rio de Janeiro, Durovni et al.,10 in a cluster randomized trial, reported a significant increase in IPT prescriptions in 29 PHC clinics for the care of HIV-infected individuals after TB screening training, with a 24% decrease in deaths from TB in this population.
Overall, the deficiency of resources for better LTBI screening in children and adolescents in primary and secondary HUs has not been evaluated in Brazil, but a poor resolution at the primary care level in the diagnosis of adult presumed TB patients attended in five municipalities has been highlighted by Villa et al.22
The WHO emphasizes that even in places with extremely limited resources, where TST and chest radiography are not available, there is no justification for not prescribing treatment for active TB or LTBI in child and adolescent contacts of adults with PT. Currently, in these situations, the WHO recommends the screening approach based on symptoms associated with active TB.3,21 Asymptomatic children are directly eligible for IPT, whereas symptomatic children require further investigation to rule out active TB before IPT is started.3
As limitations of this study, we note that the population studied included only children and adolescents treated at HMJ. Therefore, the study does not allow inferences regarding all patients with LTBI in the age range studied who reside in the state of Rio de Janeiro. Furthermore, this study has limitations inherent to studies that use a secondary data source, due to the incompleteness of basic information.
Strategies to incorporate childhood TB/LTBI in PHC should be encouraged, and prospective operational research studies should be performed with this population. Further studies should be conducted to validate our results, and new approaches that include qualitative evaluation of health services should be prioritized to identify hindering and facilitating factors for the implementation of IPT in primary and secondary HUs in countries with high TB burdens.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank the HMJ staff and Daniela Ramalho for the statistical analysis.