This was a cross-sectional study aimed to determine the prevalence of and to identify risk factors for Chlamydia trachomatis (CT) among human immunodeficiency virus (HIV)-infected women attending the acquired immunodeficiency syndrome (AIDS) clinic in the city of Manaus, Brazil, in 2009-2010. Participants answered a questionnaire containing demographic, epidemiological, and clinical data. A genital specimen was collected during examination to detect CT-DNA by hybrid capture, and blood samples were taken to determine CD4+T and HIV viral load. There were 329 women included in the study. Median age was 32 years (IQR=27-38) and median schooling was nine years (IQR=4-11). The prevalence of CT was 4.3% (95%CI: 2.1-6.5). Logistic regression analysis showed that age between 18-29 years [OR=4.1(95%CI: 1.2-13.4)] and complaint of pelvic pain [OR=3.7 (95%CI: 1.2-12.8)] were independently associated with CT. The use of condom was inversely associated with CT [OR=0.39 (95%CI: 0.1-0.9)]. The results showed that younger women who did not use condoms are at a higher risk for CT. Screening for sexually transmitted infections must be done routinely and safe sexual practices should be promoted among this population.
Chlamydia trachomatis (CT) infection in the genitourinary tract is the most prevalent bacterial sexually transmitted disease (STD) worldwide.1 Genital chlamydial infection has a huge impact on sexual and reproductive health, and it is very common in developed and developing countries.2–4 In developed countries such as the United States and the United Kingdom, CT is the most commonly diagnosed bacterial sexually transmitted infection (STI) among adolescents and young adults.5,6 In Brazil, a high prevalence of CT is observed in the female population. Screening of CT in pregnant women in Brazil showed a prevalence of nearly 10%.7,8
In general, STIs increase the risk of HIV transmission and are associated with more severe and earlier symptoms in human immunodeficiency virus (HIV) infected patients. The control of these infections represents a unique opportunity to improve reproductive health of women living with HIV.9 Both ulcerative and non-ulcerative STIs increased the risk of HIV transmission by three to ten times, depending on the type and etiology of the STD.9 HIV-infected individuals affected by an STI have increased viral load in genital secretions,10,11 thereby increasing considerably their potential of infectiousness and transmission.
Some studies showed that the prevalence of CT among HIV-infected women ranges from 2% to 10%.12–15 In Brazil, a study conducted in Rio de Janeiro found a prevalence of 3% for CT among HIV-infected women.13 The aim of the current study was to determine the prevalence of and risk factors associated with CT among HIV-infected women attending a referral center for the care of AIDS patients.
Material and methodsThis is a cross-sectional study conducted among HIV-infected women who attended the service for specialized care for AIDS at the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-DHVD), Manaus city, state of Amazonas, Brazil, from June 2009 to June 2010. HIV-infected women aged 18 to 49 years, not pregnant, and with no history of hysterectomy were invited to participate in the study after signing an informed consent. The Ethical Committee for Research of the FMT-DHVD approved all of the procedures used in the study, which was filed under the Registration No. 327-09, 06/04/2009. HIV-infected women diagnosed with CT during the study were provided treatment in accordance with the Manual of STI Control Program of the Ministry of Health of Brazil.
An individual interview for all of the participants of the study was conducted to complete a standardized questionnaire containing sociodemographical, epidemiological, and clinical data. Each participant also underwent a clinical and gynecological examination for genital specimen collection for bacterioscopy, cytology, and the detection of CT DNA by hybrid capture (QIAGEN Group Corporation). A 5mL blood sample was also collected for the determination of HIV viral load and CD4+ T-cell counts.
The sample size calculation was based on an average frequency of CT of 5% (95% CI, β=2%-8%).12–15 The required number of participants was 292. Considering a loss of 20%, the final sample size was estimated as 350.
A descriptive analysis was performed for the sociodemographic, sexual history, and behavioral covariates. The frequency distribution for qualitative variables and the median and interquartile range (IQR) for quantitative variables were calculated. The prevalence of CT was estimated along with the corresponding 95% confidence interval (CI). The chi-square test with Yates’ correction was used to assess possible associations between demographic variables and CT positivity. Fisher's exact test was applied when appropriate. Multivariate logistic regression analysis was applied to examine the independent effect of each demographic or risk variable for CT diagnosis. All variables that were moderately associated (p≤0.10) were considered for inclusion in the multivariate model. In the final analysis, variables with a non-significant association (p>0.05) were removed from the model.
ResultsA total of 329 (94%) HIV-infected women participated in the study. The median age was 32 years with an interquartile range (IQR) of 27 to 38. The median of years of schooling was nine years (IQR=4-11). The overall prevalence of CT was 4.3% (95% CI; 2.1%-6.5%).
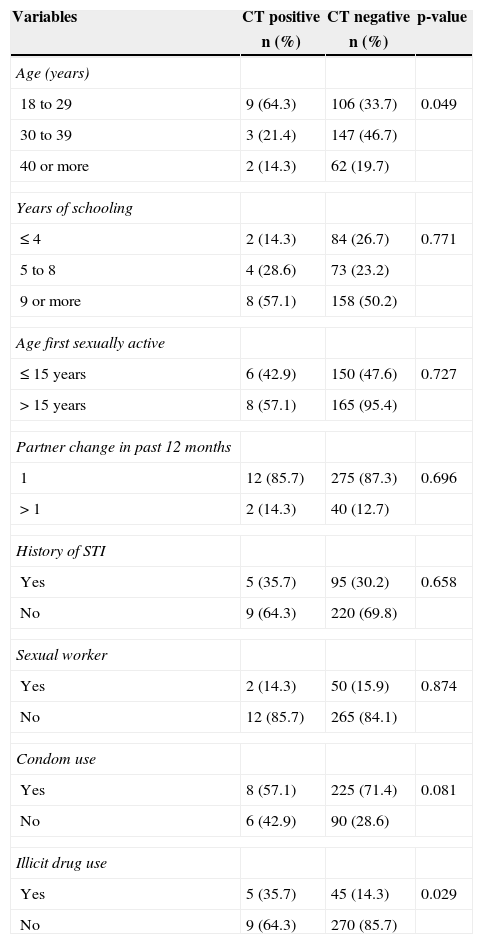
The sociodemographic and behavioral characteristics of the women in the current study are shown in Table 1. HIV-infected women with positive hybrid capture for CT were younger (18–29 years) than those with a negative test (64.3 vs. 33.7%, p=0.049). The use of illicit drugs was also more common in CT-infected than non-infected women (35.7% vs. 14.3%, p=0.029).
Demographic and behavioral characteristics of the 329 participating HIV-infected women, attendees of the service of specialized care for AIDS in Manaus, Amazonas, Brazil.
| Variables | CT positive | CT negative | p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Age (years) | |||
| 18 to 29 | 9 (64.3) | 106 (33.7) | 0.049 |
| 30 to 39 | 3 (21.4) | 147 (46.7) | |
| 40 or more | 2 (14.3) | 62 (19.7) | |
| Years of schooling | |||
| ≤ 4 | 2 (14.3) | 84 (26.7) | 0.771 |
| 5 to 8 | 4 (28.6) | 73 (23.2) | |
| 9 or more | 8 (57.1) | 158 (50.2) | |
| Age first sexually active | |||
| ≤ 15 years | 6 (42.9) | 150 (47.6) | 0.727 |
| >15 years | 8 (57.1) | 165 (95.4) | |
| Partner change in past 12 months | |||
| 1 | 12 (85.7) | 275 (87.3) | 0.696 |
| >1 | 2 (14.3) | 40 (12.7) | |
| History of STI | |||
| Yes | 5 (35.7) | 95 (30.2) | 0.658 |
| No | 9 (64.3) | 220 (69.8) | |
| Sexual worker | |||
| Yes | 2 (14.3) | 50 (15.9) | 0.874 |
| No | 12 (85.7) | 265 (84.1) | |
| Condom use | |||
| Yes | 8 (57.1) | 225 (71.4) | 0.081 |
| No | 6 (42.9) | 90 (28.6) | |
| Illicit drug use | |||
| Yes | 5 (35.7) | 45 (14.3) | 0.029 |
| No | 9 (64.3) | 270 (85.7) | |
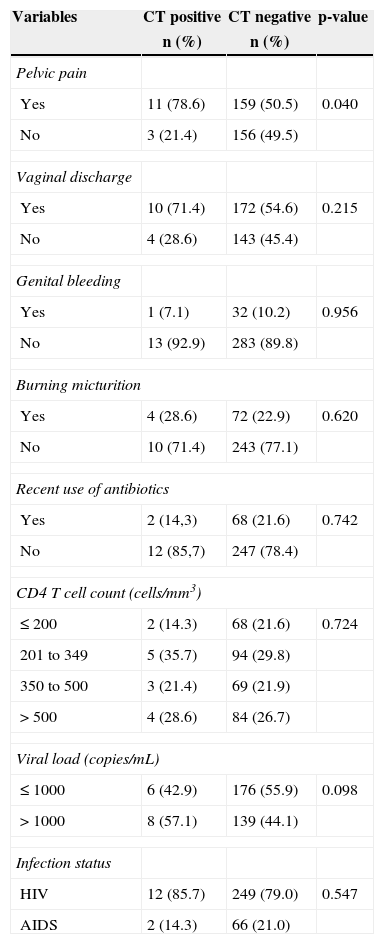
The median CD4+T cell count and viral load were 338.5 (IQR=211.5 - 513.3) cells/mm3 and 497.5 (IQR=49-11,288) copies/mm3 respectively. The clinical data of the participants are described in Table 2. A total of 261 HIV-infected women (79.3%) were classified as AIDS patients. The report of pelvic pain was more prevalent among CT-infected than non-infected women (78.6% vs. 50.5%, p=0.040).
Clinical characteristics of the 329 participating HIV-infected women, attendees of the service of specialized care for AIDS in Manaus, Amazonas, Brazil.
| Variables | CT positive | CT negative | p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Pelvic pain | |||
| Yes | 11 (78.6) | 159 (50.5) | 0.040 |
| No | 3 (21.4) | 156 (49.5) | |
| Vaginal discharge | |||
| Yes | 10 (71.4) | 172 (54.6) | 0.215 |
| No | 4 (28.6) | 143 (45.4) | |
| Genital bleeding | |||
| Yes | 1 (7.1) | 32 (10.2) | 0.956 |
| No | 13 (92.9) | 283 (89.8) | |
| Burning micturition | |||
| Yes | 4 (28.6) | 72 (22.9) | 0.620 |
| No | 10 (71.4) | 243 (77.1) | |
| Recent use of antibiotics | |||
| Yes | 2 (14,3) | 68 (21.6) | 0.742 |
| No | 12 (85,7) | 247 (78.4) | |
| CD4 T cell count (cells/mm3) | |||
| ≤ 200 | 2 (14.3) | 68 (21.6) | 0.724 |
| 201 to 349 | 5 (35.7) | 94 (29.8) | |
| 350 to 500 | 3 (21.4) | 69 (21.9) | |
| >500 | 4 (28.6) | 84 (26.7) | |
| Viral load (copies/mL) | |||
| ≤ 1000 | 6 (42.9) | 176 (55.9) | 0.098 |
| >1000 | 8 (57.1) | 139 (44.1) | |
| Infection status | |||
| HIV | 12 (85.7) | 249 (79.0) | 0.547 |
| AIDS | 2 (14.3) | 66 (21.0) | |
The final multivariate logistic regression model showed that age (18 to 29) [OR=4.1 (1.2 to 13.4)] and report of pelvic pain [OR=3.7 (1.2 to 12.8)] were independently associated with CT positivity. Condom use was inversely associated with CT [OR=0.39 (0.1-0.9)].
DiscussionThe prevalence of CT among HIV-infected women in the current study was 4.3%. This is in line with reports from Rio de Janeiro (Brazil) and Mombasa (Kenya) where a prevalence of CT of 3% and 3.2%, respectively, was observed among HIV-infected women.3,16 However, this prevalence is lower than that found among pregnant and parturient women in Brazil,7,8 and that observed (9.7%) among HIV-infected women in Thailand.15
An association of CT with younger age (18-29 years), with pelvic pain, and with unprotected sex (no condom use) was observed. Similar findings have been reported in other studies.6–8,17–19 Young women are the most heavily affected by CT. Early onset of sexual activity, having more than one partner, and practice of unprotected sex further intensify the risk of CT.20
Although cross-sectional studies are not ideal for determining risk factors, their application is justified for assessing the prevalence of and the associated factors for CT among HIV-infected women, especially at their age of highest fecundity. It is of utmost importance to demonstrate the vulnerability of this group of women to the complications of this infection in women's health. In this study, the possibility of response bias cannot be ruled out. There is always a general tendency to give socially acceptable answers. Moreover, the sensitivity of the hybrid capture test for CT is lower than the test based on nucleic acid amplification. This may lead to an underestimation of the prevalence. Conversely, the prevalence observed in the current study may be higher as HIV women seeking care at the clinics are potentially at higher risk for sexually transmitted infections.
Chlamydial infection is often asymptomatic. If CT is not detected by screening, it is unlikely to be reported. Due to the lack of specific symptoms, CT diagnosis is rarely considered. Approximately 70% to 80% of individuals with CT remain asymptomatic and undiagnosed.21 This renders the control of the spread of CT difficult as most of the asymptomatic patients are unaware of their status and do not seek treatment. The limited availability of laboratory tests for diagnosis of CT in Brazil further complicates the matter. CT can cause genitourinary infections, pelvic inflammatory disease, chronic pelvic pain, tubal-factor infertility, ectopic pregnancy and cervical cancer.19,22 Infection during pregnancy and childbirth can trigger preterm labor, ruptured membranes, and low birth weight.23,24 Infants born from mothers with CT can be infected during delivery through the birth canal and may present conjunctivitis and pneumonia.25 In patients with HIV, the co-infection with CT may prolong/or increase their potential of infectiousness, facilitating the transmission of HIV, and this epidemiological synergy may be responsible for the increased transmission of HIV in some populations.26
It is well known that when interventions in health services are effectively implemented, the quality of care is improved and consequently the condition of sexual and reproductive health, thereby avoiding complications and having positive effect in the control of the spread of STIs.27,28 The service of specialized care for HIV women must be aware of the need for STI screening in this vulnerable and high risk population.
Conflict of interestAll authors declare to have no conflict of interest.