To assess the virologic and immunological response of darunavir/ritonavir plus optimized background therapy in highly antiretroviral-experienced HIV-infected patients in Brazil.
MethodsProspective cohort study carried out in a tertiary center in Sao Paulo, Brazil. Three-class antiretroviral-experienced patients with confirmed virologic failure began darunavir/ritonavir plus optimized background therapy (nucleoside/tide reverse transcriptase inhibitors±raltegravir±enfuvirtide±maraviroc) after performing a genotypic resistance assay. Clinical evaluation and laboratory tests were collected at baseline and at weeks 12, 24, and 48. Multivariate analysis was performed to identify predictors of virologic response at 48 weeks.
ResultsNinety-two patients were included. The median of darunavir resistant mutation was 1 (range 0-6). The median genotypic sensitivity score in the optimized background therapy was 2 (interquartile range 1-2). At week 48, 83% (95% CI: 75–90%) had an HIV RNA level <50 copies/mL and the median CD4 cell count was 301 (interquartile range 224-445) cells/mm3. Baseline HIV RNA >100000 copies/mL was inversely associated with virologic success at week 48 (HR: 0.22, 95% CI: 0.06-0.85, p=0.028).
ConclusionsDarunavir/ritonavir plus optimized background therapy was a highly effective salvage regimen under clinical routine conditions in a referral center in Brazil, which is similar to the reported in high-income countries.
Highly active antiretroviral therapy (HAART) coverage has improved dramatically in low- and middle-income countries in the last years.1 However, a low proportion of patients are treated with second-line regimens in these settings.1–3 Provision of third or more-line of HAART is a major challenge to the present and future of developing countries, where there is scarce information about salvage regimens.
Darunavir/ritonavir (DRV/r) was approved by the Food and Drug Administration in June 2006 for use in treatment-experienced patients4,5 and since 2008 was included among the antiretroviral drugs available in Brazil. Efficacy and safety with DRV/r plus optimized background therapy (OBT) in treatment-experienced patients was shown in the POWER studies.6 Because of its potency, high genetic barrier and resistance profile, DRV/r is a critical component of salvage regimen. The costs of salvage regimens are high, however, there is data from high-income countries showing that DRV/r with an OBT is cost effective compared with control protease inhibitors (PIs) in highly treatment-experienced patients.7,8
DRV/r is available in Brazil and other middle-income countries, however, there is no published studies about its efficacy in this setting.
The aim of the present study was to assess, in a real clinical setting, the efficacy of DRV/r plus OBT in HIV-infected patients.
Patients and methodsStudy population and ethics statementThis prospective observational cohort study was carried out at the AIDS Clinic of the Medical School, Universidade de São Paulo, a tertiary teaching center in Brazil.
All consecutive HIV-infected adults who received at least one pill of DRV/r plus OBT between April 2008 and June 2009 were included. Criteria for DRV/r use followed the Brazilian National Guidelines to Antiretroviral Therapy: patients with confirmed virologic failure in HAART use and with a baseline genotyping showing resistance to all PIs, except DRV/r. When all PIs, including DRV/r were resistant, the PI with the best genotypic profile was selected. Thus, patients with resistance to DRV/r could be included. The HAART was selected on the basis of both the treatment history and genotyping resistance, which identify drug resistance-associated mutations in relevant regions of the HIV genome, after the recommendation of a referral physician with experience in genotyping analysis. Clinical evaluation and laboratory tests were collected at baseline and at weeks 12, 24, and 48. The laboratory adverse events were assessed on the basis of the World Health Organization toxicity grading scales.9
Laboratory evaluationsPlasma HIV-1 viral load was measured using Versant Human Immunodeficiency Virus Type 1 (HIV-1) RNA v3.0. (bDNA). The lower limit of detection was 50 copies/mL. CD4 counting was performed after staining fresh whole blood samples with labeled antibodies: CD4, CD3, CD8, and CD45 in BD TruCOUNT™ Tubes (BD Biosciences, San Jose, CA, USA). In those patients who received maraviroc, virus tropism for CCR5 co-receptor has been confirmed by the phenotypic assay Trofile® (Monogram Biosciences, South San Francisco, CA, USA). Procedures used to genotype the pol gene that codifies protease and the reverse transcriptase enzymes were available on the Brazilian National Network for HIV Genotyping (RENAGENO) website (www.aids.gov.br). We described the frequency of eleven specific DRV resistance-associated mutations according to the 2009 International AIDS Society-USA guidelines. We calculated the genotypic sensitivity score (GSS) that represents the sum of genotypic sensitivities to the drugs in the OBT, using the Brazilian Algorithm for HIV Resistance Interpretation – 2009 Database (www.aids.gov.br). The following scoring system was used for the GSS: a score of 0 was assigned if the interpretation was resistant and a score of 1 was assigned if the interpretation was sensitive. Enfuvirtide, raltegravir and maraviroc were considered to be fully active if they had not been used previously.
Statistical analysisThe primary endpoint was the percentage of patients with plasma viral load (HIV-1 RNA) below 50 copies/mL at week 48. The analysis of virologic efficacy considered non-completers as failures. The secondary endpoints were the change in CD4 cell counts from baseline through week 48 and the severe laboratory adverse events. Categorical variables were described as number (proportion) and continuous variables as median and range or interquartile range (IQR). Baseline differences between patients who reached or not viral load <50 copies/mL at week 48 were tested in a univariate analysis, which included crude odds ratios (OR), Fisher's exact test and Yates corrected Chi-squared. Independent risk factors associated with virological response at week 48 were identified in the multivariate logistic regression analysis that included variables with p<0.3 from univariate analysis. Also the Hosmer and Lemeshow goodness-of-fit test to assess the model and Wald test to assess the statistical significance of covariates were performed. The final models were derived after assessment for interaction between the relevant exposures. The value of p<0.05 was considered statistically significant. All tests were two-sided. Statistical analysis was performed by SPSS 18.0 (IBM, Chicago, IL, USA).
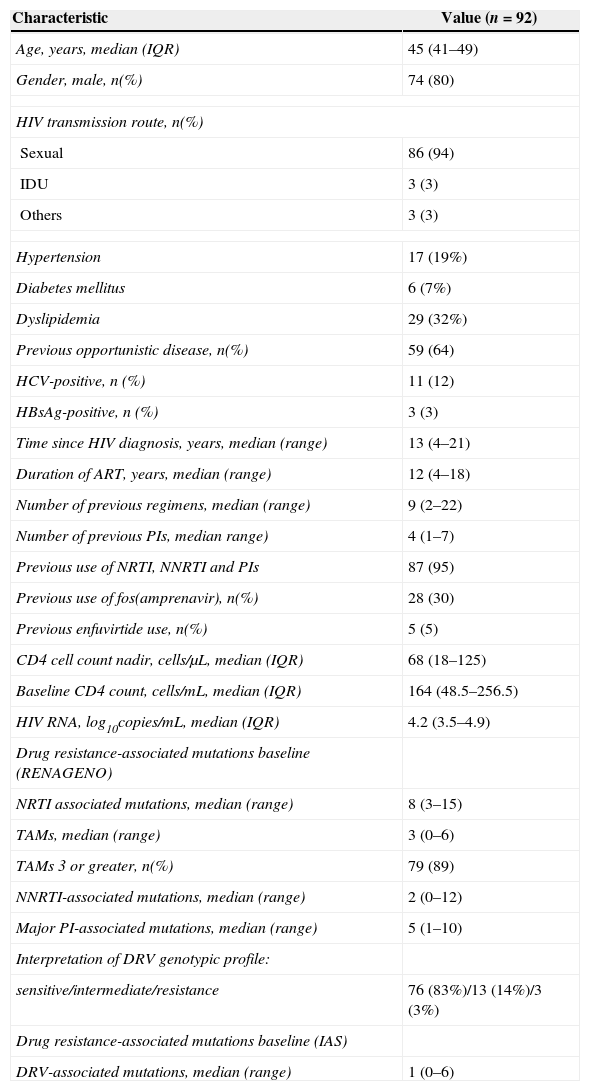
ResultsNinety two patients were included in this study and the baseline characteristics are shown in the Table 1. All patients were highly treatment-experienced with prior failure to nucleoside/tide reverse transcriptase inhibitor (NRTI), nonnucleoside reverse transcriptase inhibitor (NNRTI), and PI. The median CD4 cell count nadir (IQR) was 68 (18-125) cells/mm3 and 64% of patients presented previous opportunistic disease. Most patients had a long time of antiretroviral exposure and received a large number of regimens.
Baseline characteristics of 92 highly antiretroviral-experienced HIV-infected patients.
| Characteristic | Value (n=92) |
|---|---|
| Age, years, median (IQR) | 45 (41–49) |
| Gender, male, n(%) | 74 (80) |
| HIV transmission route, n(%) | |
| Sexual | 86 (94) |
| IDU | 3 (3) |
| Others | 3 (3) |
| Hypertension | 17 (19%) |
| Diabetes mellitus | 6 (7%) |
| Dyslipidemia | 29 (32%) |
| Previous opportunistic disease, n(%) | 59 (64) |
| HCV-positive, n (%) | 11 (12) |
| HBsAg-positive, n (%) | 3 (3) |
| Time since HIV diagnosis, years, median (range) | 13 (4–21) |
| Duration of ART, years, median (range) | 12 (4–18) |
| Number of previous regimens, median (range) | 9 (2–22) |
| Number of previous PIs, median range) | 4 (1–7) |
| Previous use of NRTI, NNRTI and PIs | 87 (95) |
| Previous use of fos(amprenavir), n(%) | 28 (30) |
| Previous enfuvirtide use, n(%) | 5 (5) |
| CD4 cell count nadir, cells/μL, median (IQR) | 68 (18–125) |
| Baseline CD4 count, cells/mL, median (IQR) | 164 (48.5–256.5) |
| HIV RNA, log10copies/mL, median (IQR) | 4.2 (3.5–4.9) |
| Drug resistance-associated mutations baseline (RENAGENO) | |
| NRTI associated mutations, median (range) | 8 (3–15) |
| TAMs, median (range) | 3 (0–6) |
| TAMs 3 or greater, n(%) | 79 (89) |
| NNRTI-associated mutations, median (range) | 2 (0–12) |
| Major PI-associated mutations, median (range) | 5 (1–10) |
| Interpretation of DRV genotypic profile: | |
| sensitive/intermediate/resistance | 76 (83%)/13 (14%)/3 (3%) |
| Drug resistance-associated mutations baseline (IAS) | |
| DRV-associated mutations, median (range) | 1 (0–6) |
IQR, interquartile range; IDU, intravenous drug users; DRV, darunavir; HCV, hepatitis C virus; ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitors; NNRTI, nonnucleoside reverse transcriptase inhibitors; PI, protease inhibitors; IAS-USA, International AIDS Society – USA; TAM, thymidine analogue mutations.
The baseline median CD4 (IQR) cell count and viral load were 164 (49-257) cells/mm3 and 4.2log10 copies/mL (3.5-4.9), respectively. The median number (range) of major PI-associated mutations was 5 (1-10), demonstrating the extensive antiretroviral agents exposure of these patients. The median number (range) of IAS DRV-associated mutations was 1 (0-6). Only 7 (7.6%) of the genotypes had ≥3 DRV-associated mutations [0 mutations=26 (28.3%); 1 mutation=37 (40.2%); 2 mutations=22 (23.9%); 3 mutations=3 (3.3%); 4 mutations=1 (1.1%); 5 mutations=2 (2.2%); and 6 mutations=1 (1.1%)]. The results of DRV genotypic evaluation using the RENAGENO algorithm were: sensitive: 76 (82.6%), intermediate resistance: 13 (14.1%), and resistance: 3 (3.3%).
All patients were naïve to DRV/r, raltegravir, and maraviroc. Five (5%) patients had previously failed to enfuvirtide. DRV/r was combined with at least one NRTI in all patients and with one or more drugs of a new class consisting of raltegravir in 66 (71.7%) cases, enfuvirtide in 55 (59.8%), and maraviroc in 8 (8.7%) of them. Tenofovir was the most frequent NRTI used (n=83, 90.2%) and its genotyping evaluation were: sensitive: 16 (17.4%), intermediate resistance: 8 (8.7%), and resistance: 68 (73.9%). The median (IQR) number of fully active drugs in the OBT was 2 (1-2) [GSS 1=31 (33.7%); GSS 2=54 (58.7%); GSS 3=7 (7.6%)]. The number and percentage of patients with one, two, three, and four fully active drugs in the salvage regimen was: 7 (7.6%), 31 (33.7%), 49 (53.3%), and 5 (5.4%), respectively. There was no difference in the proportion of patients with viral load <50 copies/mL among patients with three or two full active drugs in the salvage regimen [OR: 0.89 (95% CI: 0.22-3.23, P=1.0]. After 48 weeks, 40 (81.6%) of 49 patients with three fully active drugs and 26 (83.9%) of 31 patients with two fully active drugs in the regimen had viral load <50 copies/mL. The other 12 patients received one (n=7, 7.6%) or four (n=5, 5.4%) fully active drugs in the regimen. The baseline median CD4 (IQR) cell count was similar among patients with three or two full active drugs [152 (47-278) cells/mm3 versus 163 (42-258) cells/mm3, respectively (p=0.816)]. In addition, the baseline median viral load was similar among patients with three or two full active drugs [4.3 (3.7-4.8)log10 copies/mL versus 4.5 (3.3-5.1)log10 copies/mL, respectively (p=0.778)].
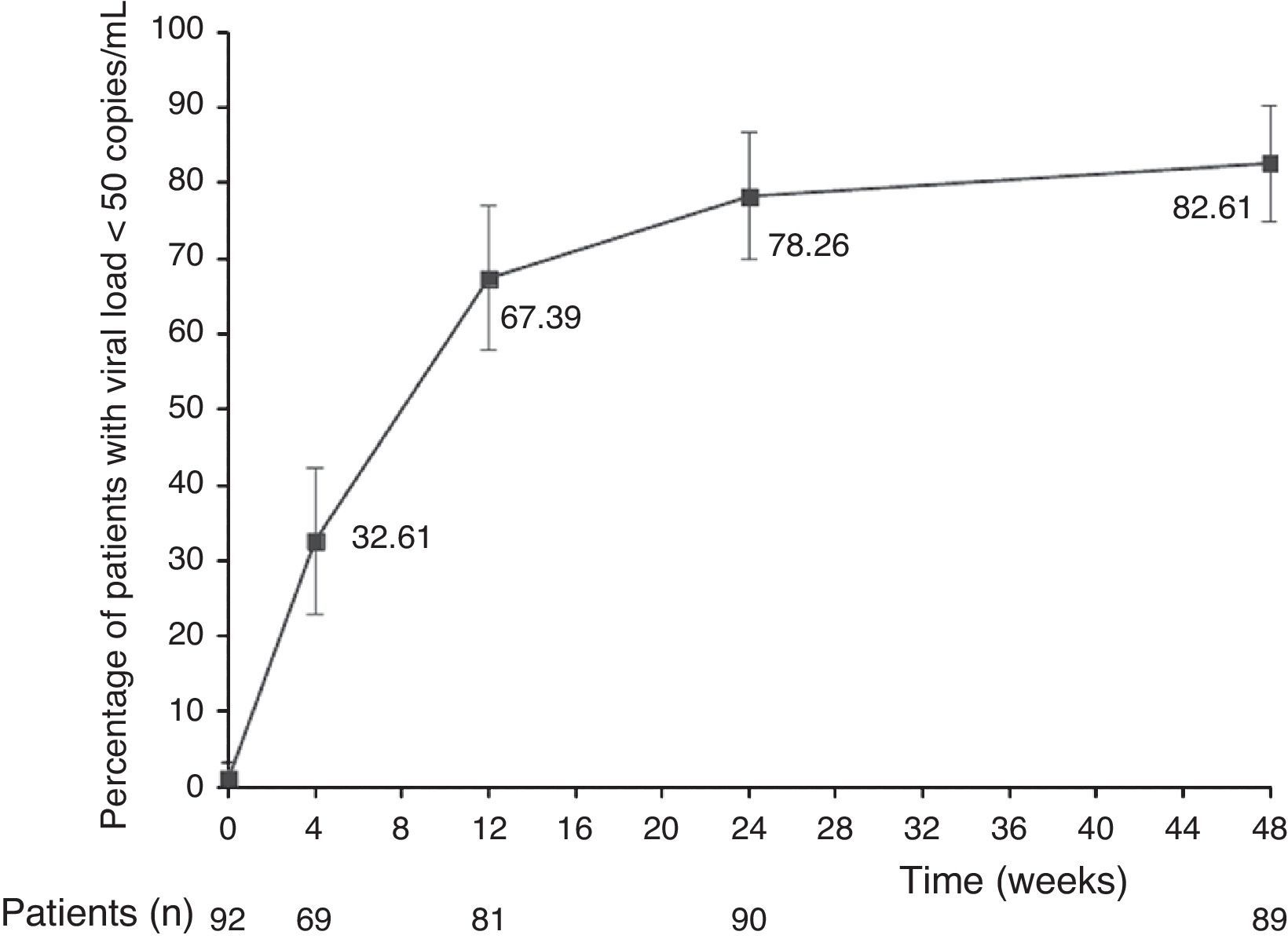
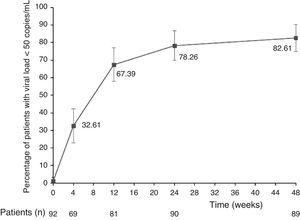
The main finding of this study was the high rate of virologic suppression with DRV/r plus OBT among highly antiretroviral-experienced HIV-infected patients. After 48 weeks, 76 de 92 patients (82.6%, 95% CI: 74.9–90.4) had viral load <50 copies/mL. Fig. 1 shows treatment efficacy during the follow-up.
After 48 weeks, the median of the CD4 cell count (IQR) was 301 (224-445) cells/mm3. CD4 cell count increased significantly by a median (IQR) of 82 (26-124) cells/mm3, 108 (54-174) cells/mm3, and 118 (52-215) cells/mm3 at weeks 12 (n=81), 24 (n=87), and 48 (n=89), respectively (p<0.0001 for all differences in comparison with baseline). At week 12, CD4 cell count was not available in 11 patients. Two of them had died and in 9 (10%) cases the samples were not collected timely. At week 24, CD4 cell count was not available in 5 patients. Two of them were of patients who died within 12 weeks, and in 3 cases, the samples were not collected timely. At week 48, the three lost samples were of patients who died, including one after week 24.
Three (3.3%) patients died during the study period. The first one died at day 3 of salvage regimen due to progression of steatohepatitis, cholestasis and pancreatitis caused by cytomegalovirus and HIV. The second patient died at week 4 of salvage regimen (CD4 cell count=110 cells/mm3 and viral load <50 copies/mL) due to progression of Burkitt's lymphoma, sepsis and intestinal perforation. The third case died at week 32 of salvage regimen (CD4 cell count=439 cells/mm3 and viral load <50 copies/mL) due to acute liver failure in a prior idiopathic cirrhotic patient. In this last case, death was probably related to salvage regimen.
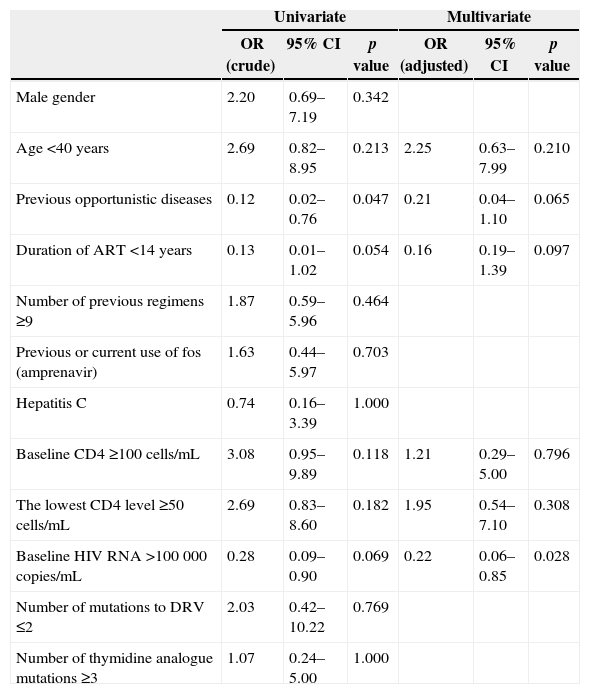
Univariate and multivariate analysis of factors associated with HIV RNA <50 copies/mL at 48 weeks of HAART are shown in the Table 2. Baseline HIV RNA >100000 copies/mL was inversely associated with virologic success.
Univariate and multivariate analysis of factors associated with HIV RNA <50 copies/mL at week 48 of antiretroviral therapy.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR (crude) | 95% CI | p value | OR (adjusted) | 95% CI | p value | |
| Male gender | 2.20 | 0.69–7.19 | 0.342 | |||
| Age <40 years | 2.69 | 0.82–8.95 | 0.213 | 2.25 | 0.63–7.99 | 0.210 |
| Previous opportunistic diseases | 0.12 | 0.02–0.76 | 0.047 | 0.21 | 0.04–1.10 | 0.065 |
| Duration of ART <14 years | 0.13 | 0.01–1.02 | 0.054 | 0.16 | 0.19–1.39 | 0.097 |
| Number of previous regimens ≥9 | 1.87 | 0.59–5.96 | 0.464 | |||
| Previous or current use of fos (amprenavir) | 1.63 | 0.44–5.97 | 0.703 | |||
| Hepatitis C | 0.74 | 0.16–3.39 | 1.000 | |||
| Baseline CD4 ≥100 cells/mL | 3.08 | 0.95–9.89 | 0.118 | 1.21 | 0.29–5.00 | 0.796 |
| The lowest CD4 level ≥50 cells/mL | 2.69 | 0.83–8.60 | 0.182 | 1.95 | 0.54–7.10 | 0.308 |
| Baseline HIV RNA >100000 copies/mL | 0.28 | 0.09–0.90 | 0.069 | 0.22 | 0.06–0.85 | 0.028 |
| Number of mutations to DRV ≤2 | 2.03 | 0.42–10.22 | 0.769 | |||
| Number of thymidine analogue mutations ≥3 | 1.07 | 0.24–5.00 | 1.000 | |||
ART, antiretroviral therapy; DRV, darunavir; GSS, genotypic sensitivity score. Binary logistic model in which variables with p<0.3 in univariate analysis entered.
All the 55 patients who used enfuvirtide had at least one sign or symptom characterizing local injection site reactions. In most cases these reactions were mild but 6 (10.9%) patients discontinued this drug during the study period because presented moderate or severe reactions. Two of them had switched from enfuvirtide to raltegravir. All of these 6 patients maintained a salvage regimen with two or more fully active drugs, including those sensitive to DRV/r. Five of these six patients had viral load <50 copies/mL at week 48.
We found grade 3-4 laboratory abnormalities in 9 (9.8%) patients: elevated glucose level=3 cases; elevated triglycerides levels=2 cases; elevated alanine or aspartate aminotransferase level=2 cases; and creatine phosphokinase level=1 case. We observed two laboratory abnormalities that resulted in the tenofovir discontinuation: proximal tubulopathy in one person and hepatotoxicity in the other one. In the last case all drugs (tenofovir, lamivudine, DRV/r, raltegravir and maraviroc) were discontinued.
DiscussionThis study shows feasibility to achieve excellent virologic and immunologic response with DRV/r plus OBT among highly antiretroviral-experienced HIV-infected patients under routine clinical care in Brazil.
A recent systematic review and meta-analysis of randomized controlled trials to assess the overall efficacy of new antiretroviral drugs, including DRV/r, confirmed the benefit of these drugs in treatment-experienced patients, compared with placebo.10
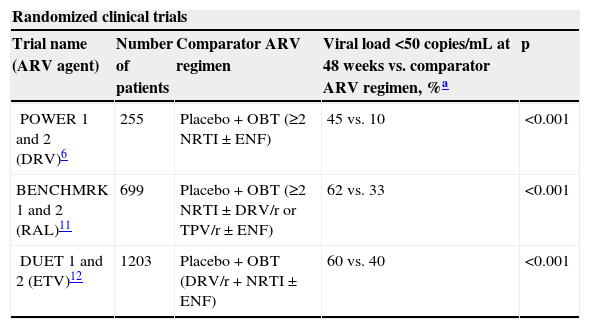
Our results should be interpreted in the context of the prior studies with DRV/r. Main information of selected randomized, non-randomized clinical trials, and observational studies that evaluated DRV/r as part of a salvage regimen in HIV-infected patients are shown in Table 3. These studies showed the importance of DRV/r as part of a salvage regimen.
Selected randomized, non-randomized clinical trials and observational studies of salvage regimens including darunavir/ritonavir (DRV/r).
| Randomized clinical trials | ||||
|---|---|---|---|---|
| Trial name (ARV agent) | Number of patients | Comparator ARV regimen | Viral load <50 copies/mL at 48 weeks vs. comparator ARV regimen, %a | p |
| POWER 1 and 2 (DRV)6 | 255 | Placebo+OBT (≥2 NRTI±ENF) | 45 vs. 10 | <0.001 |
| BENCHMRK 1 and 2 (RAL)11 | 699 | Placebo+OBT (≥2 NRTI±DRV/r or TPV/r±ENF) | 62 vs. 33 | <0.001 |
| DUET 1 and 2 (ETV)12 | 1203 | Placebo+OBT (DRV/r+NRTI±ENF) | 60 vs. 40 | <0.001 |
| Non-randomized clinical trial | ||||
|---|---|---|---|---|
| Trial name | Number of patients | Regimen | OBT | Viral load <50 copies/mL at 48 weeks, % |
| TRIO13 | 103 | DRV/r+ETV+RAL | ±NRTI±ENF | 86 (95% CI: 80-93) |
| Observational studies | ||||
|---|---|---|---|---|
| Number of patients | Regimen | OBT | Viral load <50 copies/mL, % | |
| Imaz et al.14 | 32 | DRV/r+ETV+RAL | None | 94 (at 24-week) |
| Imaz et al.15 | 122 | At least 3 of DRV/r, ETV, RAL, MVQ | ±NRTI | 78 (at 48-week) |
| Delaugerre et al.16 | 62 | 2 ITRN+DRV/r | At least 1 of RAL, ETV or ENF | 55 (at 36-week) |
ARV, antiretroviral; DRV, darunavir; RAL, raltegravir; ETV, etravirine; MVQ, maraviroc; ENF, enfuvirtide; OBT: optimized background therapy; NRTI, nucleoside reverse-transcriptase inhibitor; IC, confidence interval.
In the present study we observed 83% of patients with viral load <50 copies/mL at week 48. This outcome is superior to randomized clinical trials6,11,12 but similar to one non randomized clinical trial13 and some observational studies.14,15 Although comparisons between studies should be made with caution due to design issues different enrollment criteria, patient population, time period, and composition of the regimen (Table 3), we consider that some characteristics seem to justify our results. All of our multi-experienced patients were naïve both to DRV/r and raltegravir and only 5% had prior failure with enfuvirtide. All received at least one drug of a new class (raltegravir, 72% of cases; enfuvirtide, 60% of cases; and maraviroc, 9% of cases), and the median (IQR) GSS in the OBT was 2 (1-2). Thus, 92% of patients received at least two active drugs, most of them including a DRV/r and a new active drug. In addition, most of patients (92%) showed genotypes with <3 DRV-associated mutations, showing viral susceptibility to this antiretroviral agent. Differential features were observed in randomized clinical trials and could explain, at least in part, the variable efficacy rates. In POWER 1 and 2 studies,6 enfuvirtide was the single new drug available and 78% of DRV/r patients showed <3 DRV resistance-associated mutations. In BENCHMRK 1 and 2 studies,11 85% patients had ≥1 active agents in the OBT by phenotypic sensitivity score (PSS), and 38% and 40% of raltegravir group received enfuvirtide and DRV/r, respectively. In DUET 1 and 2 studies,12 84% patients had ≥1 active agents in OBT by PSS. As all patients from both groups received DRV/r, the relatively high efficacy rate in the placebo group is in accordance with the efficacy observed in the POWER trials.6 Our results are similar to ANRS TRIO Trial, a multicenter, phase II, non-comparative study,13 in which genotypic susceptibility to DRV/r and etravirine was an inclusion criterion and all patients were naïve to raltegravir. This profile was fundamental to explain the high rate of virology success of this trial. In addition to randomized and clinical trials, the results of two observational studies are in line with the expected benefit of DRV/r-based salvage regimens. In the first,14 65% of the patients received three full active drugs, without taking into account the NRTIs used, and all patients received at least two fully active new drugs in the regimen. Baseline median (range) DRV resistance-associated mutations was 1 (0-2). In the second,15 all patients received DRV/r, etravirine and raltegravir, and baseline median (range) DRV resistance-associated mutations was 1 (0-3). Thus, similar to our study, composition of the salvage regimens and viral susceptibility to DRV/r were favorable and probably influenced the high rates of efficacy. By the contrast, the reported efficacy of another observational study was moderate.16 Some features could partially to explain its efficacy results: the baseline median (IQR) DRV resistance-associated mutations was 2 (1-3), only 47% of the patients received at least one new active drug and 32% of DRV/r patients showed≥3 DRV resistance-associated mutations.
Taken together, the optimal composition of salvage regimen and resistance profile of most of our patients seem to explain the high rate of virologic suppression observed in the present study even similar with a rate of virologic suppression to that expected in treatment-naïve patients.
Most of our patients received a new scheme in accordance with current recommendations of main international guidelines to salvage regimen with at least two, and preferably three, fully active drugs on the basis of drug treatment history, resistance testing, or new mechanistic class.17,18
Several subgroup analyses identified higher CD4 cell count and lower viral load associated with better virologic response in salvage regimen.6,11,19 In this line, in the present study, only baseline viral load >100000 copies/mL was inversely related with viral load <50 copies/mL at week 48.
There is no information from randomized clinical trials designed to answer the question about the use of two versus three full active drugs in salvage regimen. A recent systematic review and meta-analysis identified that the main predictive factor for efficacy was the number of fully active drugs.10 However, this study did not have appropriate data to evaluate potential benefits of a regimen with three full active drugs. Subgroup analyses of randomized clinical trials showed a trend to benefit of schemes with three versus two active drugs.6,11,12,19 In addition, published non-randomized trial and observational studies with three active drugs reported very high rates of virologic suppression.13–15 In our exploratory analysis, differences in the proportion of patients with viral load <50 copies/mL among patients with three or two full active drugs were not observed. Currently, it is common to recommend giving three fully active drugs to most patients with multiclass drug resistance in routine clinical practice from developed countries.17,18,20,21 However, it is a controversial issue and an individually based approach is necessary, also taking into account the patient's needs and tolerance to previous regimens, complexity of the salvage therapy and costs.22 This last issue is particularly challenging in developing countries where cost-effectiveness analyses of salvage regimens are awaited.
The number of active drugs is an important component but this variable should be always evaluated in the context of the drug potency and viral susceptibility.18 It is probable that in some subsets of patients with factors associated with viral response (for example, high CD4 cell counts, low basal viral load and/or absence of or few DRV-associated resistance mutations) a DRV/r-based salvage regimen employing only two full active drugs might be as effective as three full active drugs.22,23
Two studies performed in Sao Paulo reported a low prevalence of DRV resistance-associated mutations in HIV-infected patients presenting virologic failure and without history of DRV exposure. In one, only 6% of 171 HIV-infected patients failing PI-based regimes showed genotypes with ≥3 DRV resistance-associated mutations.24 In the other one, only 2% of 2474 HIV-infected patients failing several antiretroviral regimes showed genotypes with ≥3 DRV resistance-associated mutations.25 These results suggested that DRV could be an important component of a salvage regimen in our setting and the present study confirmed this hypothesis.
In our study, enfuvirtide was used in 60% of cases. Nowadays, the higher accessibility of new generation and new classes of drugs will prevent the extensive use of this one.17,18,21,26
There is a concern about the cross-resistance profile between DRV and fos(amprenavir). Some studies suggested that prior failure27 or prior use24 of fos(amprenavir) were associated with having more DRV-specific resistance mutations in multivariate analysis. Nevertheless, in the present study, previous or current use of fos(amprenavir) was not associated with virologic success at week 48 of HAART. This finding should be considered with caution. The number of patients included in this study does not confer statistical power to answer this question. Although the results of POWER 1, 2, and 3 subgroup analyses reported that prior utilization or resistance to fos(amprenavir) at screening had only a minimal effect on the virologic response to DRV/r at week 48,28 this issue is not completely resolved.
The results of the present study indicate the tangible option to treat highly-experienced HIV-infected patients in low- and middle-income countries. However, this study presents some limitations. First, although the observational nature of this study precludes some conclusive results, our study included all consecutive HIV-infected adults who received at least one pill of DRV and the analysis of virologic efficacy considered non-completers as failures. Second, these results cannot be necessarily extrapolated to other settings. Our patients were treated in an urban teaching center of a country with a well-structured National Program of AIDS, including a free and universal access to ART and a network of laboratories to perform CD4 cell count, HIV-1 viral load, and genotyping. Third, patients of the present study were included in the first months of availability of DRV and raltegravir in our city. This factor can explain, at least in part, the high frequency of sensitivity to these drugs and the consequent high efficacy in our patients. A longer period of inclusion could allow a higher number of patients but particularly a more heterogeneous population, including cases with more resistance virus and patients with less therapeutic options. Future studies will be important to assess the potential relation among temporal trends and the value of salvage regimen in our setting.
In conclusion, DRV/r plus OBT was highly effective and well-tolerated in highly antiretroviral-experienced patients, under clinical routine conditions, similar to reported in high-income countries. Strong and structured National Programs and continued international efforts are necessary in the ongoing success of access to HIV care strategies in low- and middle-income countries, particularly provision of third or higher-line of HAART.
Conflict of interestJEV has received honoraria for lectures and/or travel grants from Abbott, Boehringer Ingelheim, Bristol-Meyer-Squibb, GlaxoSmithKline, Janssen-Cilag, Roche, and Merck Sharp & Dohme. Other authors: none declared.
Ethical approvalThe study protocol was approved by the Institutional Review Board of Universidade de São Paulo and informed consent was obtained from all patients.
The authors would like to thank the nurses and physicians involved in the care of the patients included in this study, and two anonymous referees for valuable comments and suggestions.