A 72-year-old, female, Greek patient presented to the Emergency Department of our hospital complaining of fever and fatigue. She also mentioned that she had recently returned from a trip to the Republic of Congo, Africa. Laboratory evaluation revealed pancytopenia, abnormal liver tests, elevated lactate dehydrogenase (LDH) and high inflammation markers (C-reactive protein and erythrocyte sedimentation rate). She was admitted for further investigation.
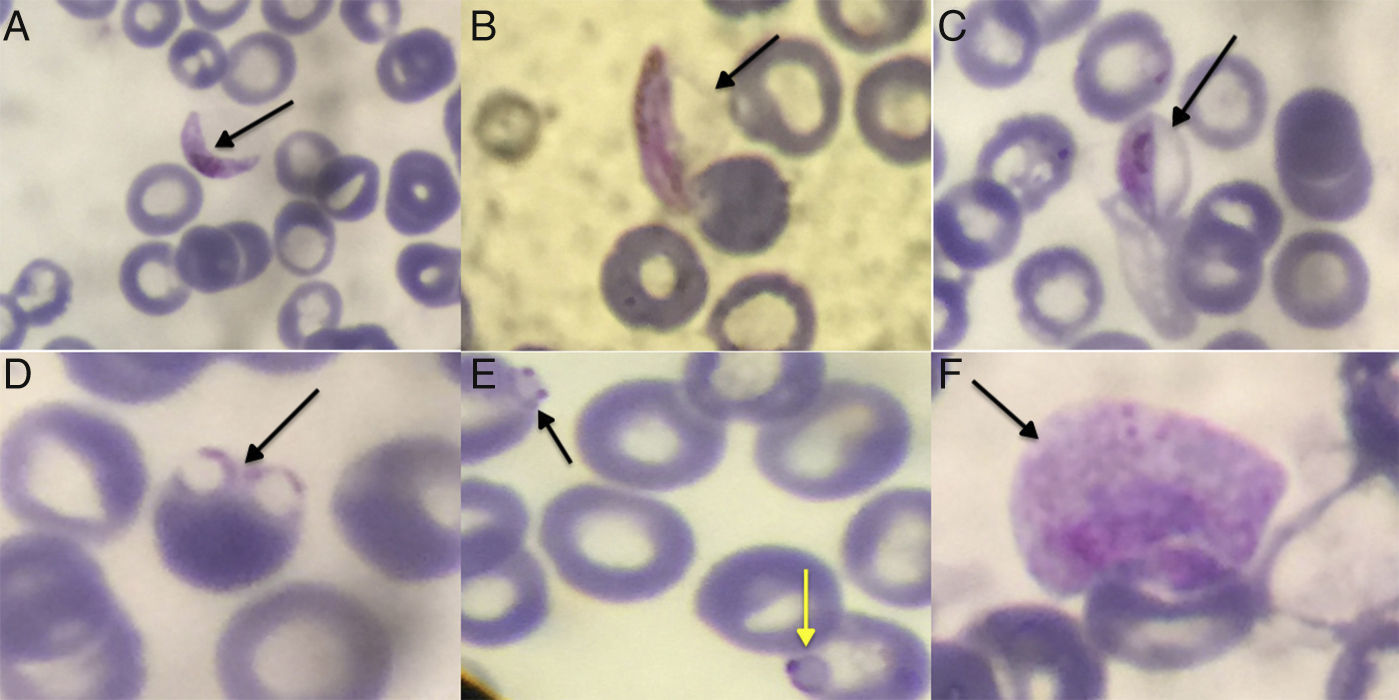
Due to the recent trip to Congo, our differential diagnosis included malaria, which is endemic in the region. A search for characteristic forms of malaria parasite in thick drop and thin layer of peripheral blood with May-Grunwald/Giemsa stain was performed. Examination of thin layer revealed crescent elongate gametes (Fig. 1A–C) and red blood cells (RBC) with delicate ring form trophozoites. Some RBC were multiple-infected, or they had rings with double chromatin dots (Fig. 1D and E), while the level of parasitemia was low (<0.1%). These findings pointed toward a Plasmodium falciparum (P. falciparum) infection,1 a species endemic in the area visited by the patient and causing more than 90% of malaria cases.2 At the same time, we performed a rapid diagnostic test (RDT), a lateral flow immuno-chromatographic antigen-detection test, that relies on the capture of dye-labeled antibodies to produce a visible band on a strip. Antigens detected were histidine rich protein-2 (HRP-2), specific for P. falciparum, and Plasmodium lactate dehydrogenase (pLDH), which is present in the rest of the species with 90–92% identity to pLDH from P. falciparum. RDT is considered to be useful to distinguish P. falciparum from non-falciparum infections. In our case, RDT was positive for both antigens with two bands visible on the strip. Given that malaria infection is under mandatory surveillance by the national infection control center, a sample as well as the smear and photos of the findings were sent to a reference laboratory for confirmation of the infection with molecular testing. The initial polymerase chain reaction (PCR) was positive for P. falciparum and negative for Plasmodium vivax (P. vivax). However, thorough observation of the photos, brought to light pale RBC larger than normal, with an irregular coating, amoeboid trophozoite, and granulation therein (Fig. 1F). These features raised the suspicion of coinfection with Plasmodium ovale (P. ovale)2 and specific PCR performed yielded positive results. Initial, empiric treatment of the patient included the combination of doxycycline and clindamycin. After laboratory confirmation of malaria diagnosis, the therapy was changed to a 3-day course of atovaquone and proguanil. The patient fully recovered, while no complications were observed.
Thin blood smear: (A) P. falciparum gametocyte (crescent or sausage shaped). The red chromatin and pigment is more coarse and concentrated in the macrogametocytes than the microgametocytes. (B) Gametocyte of P. falciparum showing the membrane of the RBC. Sometimes in thin blood smears, the remnants of the host RBC can be seen; this is often referred to as Laveran's bib. (C) Gametocyte of P. falciparum stage III. (D) Rings of P. falciparum (multiple-infected RBC). (E) Double chromatin dot in the infected RBC (black arrow) and the appliqué form in the infected RBC – accolées (yellow arrow) of P. falciparum. (F) Pale and larger than normal erythrocyte with parasitism, with amoeboid trophozoite and Schuffner's dot within with outline irregular. This refers to P. ovale.
The presence of P. falciparum represents a medical emergency, even with very low parasitemia, as it is the species associated with higher morbidity.3 Malaria is a treatable disease after early detection and identification of the species. The gold standard for the diagnosis remains blood smear test that enables discrimination of species and quantification of infection. However, microscopy is time consuming, and requires visualization of at least 500 fields, and vast experience. In conclusion, despite the progress of the diagnostic techniques, the laboratory diagnosis of malaria remains a challenge, especially in non-endemic areas of the world, where physicians’ relative experience is limited. High degree of clinical suspicion and close collaboration between clinicians and the laboratory are essential factors for the prompt diagnosis of the disease.
Conflicts of interestThe authors declare no conflicts of interest.