Enterotoxigenic Escherichia coli (ETEC), a major cause of diarrhea in children under 5, is an important agent for traveler's diarrhea. Heat-labile enterotoxin (LT) and colonization factors (CFs) are two main virulence mechanisms in ETEC. CS6 is one of the most prevalent CFs consisting of two structural subunits viz., CssA, CssB, necessary for attachment to the intestinal cells.
MethodsIn the present research, a chimeric trivalent protein composed of CssB, CssA and LTB was constructed. The chimeric gene was synthesized with codon bias of E. coli for enhanced expression of the protein. Recombinant proteins were expressed and purified. Mice were immunized with the recombinant protein. The antibody titer and specificity of the immune sera were analyzed by ELISA and Western blotting. Efficiency of the immune sera against ETEC was evaluated.
ResultsAntibody induction was followed by immunization of mice with the chimeric protein. Pretreatment of the ETEC cells with immunized animal antisera remarkably decreased their adhesion to Caco-2 cells.
DiscussionThe results indicate efficacy of the recombinant chimeric protein as an effective immunogen, which induces strong humoral response as well as protection against ETEC adherence and toxicity.
Enterotoxigenic Escherichia coli (ETEC) is one of the important agents causing diarrhea in children under five years old in developing countries and is a major cause of diarrhea in travelers to those regions.1,2 This microorganism is responsible for 400,000 to 800,000 deaths per year.3 ETEC strains have several virulence factors like heat-labile enterotoxin (LT), heat-stable enterotoxin (ST) and colonization factors (CFs).1 CFs are surface proteins that mediate adherence to intestinal mucosa, the first step to begin pathogenesis.4 Following adherence, either or both LT or ST are expressed, resulting in diarrheal disease.5
Of more than 25 identified CFs,6 CS6 is one of the most epidemiologically prevalent CF in many countries, often accounting for up to 20–30% of all clinical isolates.1,4 This has led to interest to try to use CS6 alone or in combination with other antigens in an ETEC vaccine.1,7 The morphology of CS6 is non-fimbrial and is expressed either alone or with CS5 or less commonly with CS4 on ETEC strains.7,8
The genes associated with CS6 are expressed as a typical bacterial operon, consisting of four genes viz., cssA, cssB, cssC, and cssD.7 CssA and CssB are structural subunits, whereas CssC and CssD are recognized to be chaperone and usher respectively.9 CssC protein assists in the folding of the CssA and CssB structural subunits, and CssD has been ascribed an usher function responsible for transport of CssA and CssB to the cell surface.7 40kDa CssA and 15.5kDa CssB are necessary for attachment to intestinal cells.4,7 The CssA interacted with the host cell fibronectin while CssB binds to cell surface sulfatide.4,10 Studies have indicated that antibodies against both structural subunits are essential to inhibit the adhesion.1,4
LT toxin consists of a pentameric ring of five identical binding (LTB) subunits of 11.5kDa surrounding an active subunit (LTA) and bind strongly to the intestinal receptor GM1 on the surface of eukaryotic cells.11,12 The B subunit mostly associates with immunity against LT and induces both systemic and mucosal antibody responses.13 Since most clinical ETEC isolates can produce LT and has adjuvant properties, therefore it is a suitable candidate antigen to provide anti-LT immunity.14,15
In order to provide protection, an ETEC vaccine should enclose the most prevalent CF and a LT toxoid or B subunit of LT toxin until it blocks adherence and toxin activity.5,16
Because live attenuated or killed bacterial cell vaccines are potentially associated with transient side effects, use of subunit vaccines has been preferred. The subunit vaccines are considerably safe immune system stimulators with the ability to target the site where immunity is required.17
In this study a trivalent recombinant protein was designed against ETEC CS6 strains. A construction containing structural subunits of CS6 (CssB, and 100 amino acids from the C-terminal of the protein CssA) and B subunit of LT toxin was designed. The immunogenicity of a trivalent recombinant protein coded by a synthetic gene is reported.
Materials and methodsBacterial strains, plasmids and mediaETEC strains were isolated from children with diarrhea. The strains were previously characterized for the presence of CFs and enteroxins and used for challenge purpose. Plasmids pET-28a and pET-32a were from Novagen (USA). E. coli BL21 (DE3), DH5α and Top10 were procured from Pasteur Institute of Iran. The E. coli strains were grown in Luria–Bertani (LB) broth at 37°C.
In silico predictions and construction of chimeric geneChimeric gene was designed based on the nucleotide sequence of ltb, cssA and cssB genes (Gen Bank accession numbers; M17874.1, GQ241328.1, UO4844.1, respectively). The sequences were used to generate a chimeric trivalent protein fused together by (EAAAK)4 hydrophobic linkers in order to find the best epitope exposing chimeric antigen. The in silico gene analysis and multi parameter gene optimization of the synthetic chimeric gene was performed using OPTIMIZER server. Itasser and phyre software were used in order to estimate tertiary structure prediction. The chimeric proteins were analyzed for B-cell epitopes using Bcepred server. The gene encoding protein was synthesized by Shine Gene Molecular Biotech, Inc. (Shanghai, China).
Expression and purification of chimeric proteinThe synthetic gene (cssA- cssB -ltB) subcloned into pET28a was transformed to E. coli BL21 (DE3) (Novagen). The transformants were grown in Luria Bertani (LB) broth supplemented with 30μg of kanamycin/ml. Expression of the chimeric protein was induced at OD600 of 0.5 by addition of 1mM isopropyl-β-d-galactopyranoside (IPTG) and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Batch purification of His-tagged proteins from E. coli under denaturing conditions (Qiagen) was carried out and validated by SDS-PAGE. The denaturant (8M urea) was removed by stepwise dialysis.
Western blot analysisThe proteins separated by SDS-PAGE were blotted onto nitrocellulose membrane using transfer buffer (39mM glycine, 48mM Tris–base, 0.037% SDS, and 20% methanol). The membrane was incubated in 10ml blocking buffer (5% skim milk in PBST [137mM NaCl, 2.7mM KCl, 4.3mM Na2HPO4 and 0.05% (v/v) Tween-20]) at 4° C overnight. The membrane was then incubated in a 1:10,000 dilution of mice anti-His-tag -conjugated HRP and anti-CTX (Sigma) in the PBST, with mild shaking for 1h at 37°C. Detection was carried out using HRP staining solution containing 3,3′-diaminobenzidine (DAB) (Sigma) and H2O2.
Animal immunizationFive-week old female BALB/C mice and New Zealand White female rabbits procured from Pasteur Institute of Iran were randomly divided into test and control groups. Animals were immunized four times. Each mouse received 20μg recombinant CS6 protein along with complete Freund's adjuvant (SIGMA) subcutaneously. Booster doses of 15 and 10μg recombinant proteins with incomplete Freund's adjuvant were injected after 20 and 35 days, respectively. PBS served as control in parallel to the aforementioned procedure. The sera taken from animal blood after the second and third injections were stored at −70°C for further analyses.
Determination of serum IgG responses to recombinant proteinsIndirect ELISA was used for antibody titration. A 96-well ELISA plates (Nunc) were coated with 5μg of CssA, CssB and LTB proteins in coating buffer (64mM Na2CO3, 136mM NaHCO3, pH 9.8) at 4°C overnight. The plates were washed three times with PBST and the non-specific sites were blocked with skim milk solution 5% (w/v) in PBST. The serum samples serving as primary antibodies were serially diluted to 1:500 in PBST and added to the ELISA plates with incubation at 37°C for 45min. The plates were washed thrice in PBST and Goat Anti-Mouse IgG Antibody (12,000 in PBST) was added to the ELISA plates as the secondary antibody. The plates were incubated for 30min at 37°C and were then washed. The wells added with 100μl of citrate buffer containing 0.06% (W/V) of O-phenylene diamine dihydrochloride (OPD) (SIGMA) and 0.06% (V/V) hydrogen peroxide were incubated at room temperature for 15min. The reaction was stopped with 100μl of 2M H2SO4 and the OD492 was read on a microplate reader (Bio-Rad).
ETEC binding inhibition assayCaco-2 cells (104/well) were added and grown to confluence in 96-well plates with DMEM supplemented with 10% (v/v) FBS at 37°C in a culture flask. ETEC strain was grown in LB broth. The bacterial suspension (105/well) was pretreated with 40μl of immunized mice antisera for 15min at room temperature. The bacterial mixture was added to Caco-2 cells and incubated for 2h at room temperature. The supernatant was decanted, and the cells were washed with PBS. After digestion, the suspended bacteria were serially diluted and incubated in LB agar at 37°C for 16h. Subsequently, the number of bacteria adhered to the Caco-2 cells was counted. The sera of non-immunized mice were used as control.
Anti-LT antibody neutralizationThe production of LT toxin neutralizing antibodies was performed with rabbit ileal loop, cAMP immunoassay and Y-1 cell rounding assay. The rabbit ileal loop test was carried out according to the methods described by De and Chatterjee.18 New Zealand White rabbits (1.5kg) were fasted for 24h before surgery. The rabbits were anesthetized by the intramuscular injection of fentanyl (SIGMA) (0.3ml/kg of body weight) and zolazepam chloridrate (0.4ml/kg of body weight). 10-cm ligated ileal loops were constructed. ETEC at 1×108 concentration were incubated with mouse serum raised against the chimeric protein, for 30min. ETEC bacteria incubated with non-immune mouse serum served as negative control. The above bacterial mixture and PBS were inoculated into each ligated loop using a 25-gauge needle. After 18h, the animals were sacrificed with intravenous 3% pentobarbital, and zolazepam chloridrate (0.4ml/kg). For each ileal loop, the volume of secretion was measured after excision. Experimental infection of the rabbits was performed at the Faculty of Veterinary Medicine, University of Tehran. Research was conducted in compliance with the Animal Welfare Act and regulations related to experiments involving animals. The production of antibodies that neutralized LT toxin was also performed with a cAMP immunoassay kit (Abnova) and CHO cells. CHO cells were seeded in 24-well sterile culture plates at a density of 5×104 cells per well and were grown overnight to approximately 80% confluence. 0.1μg of LT was incubated with 50μl mice serum at room temperature for 1h, and the mixture was added to each well for incubation at 37°C in 5% CO2 for 2h After washes, the cells were lysed with 0.1M HCl and the supernatant was used in cAMP assay following the manufacturer's protocol.
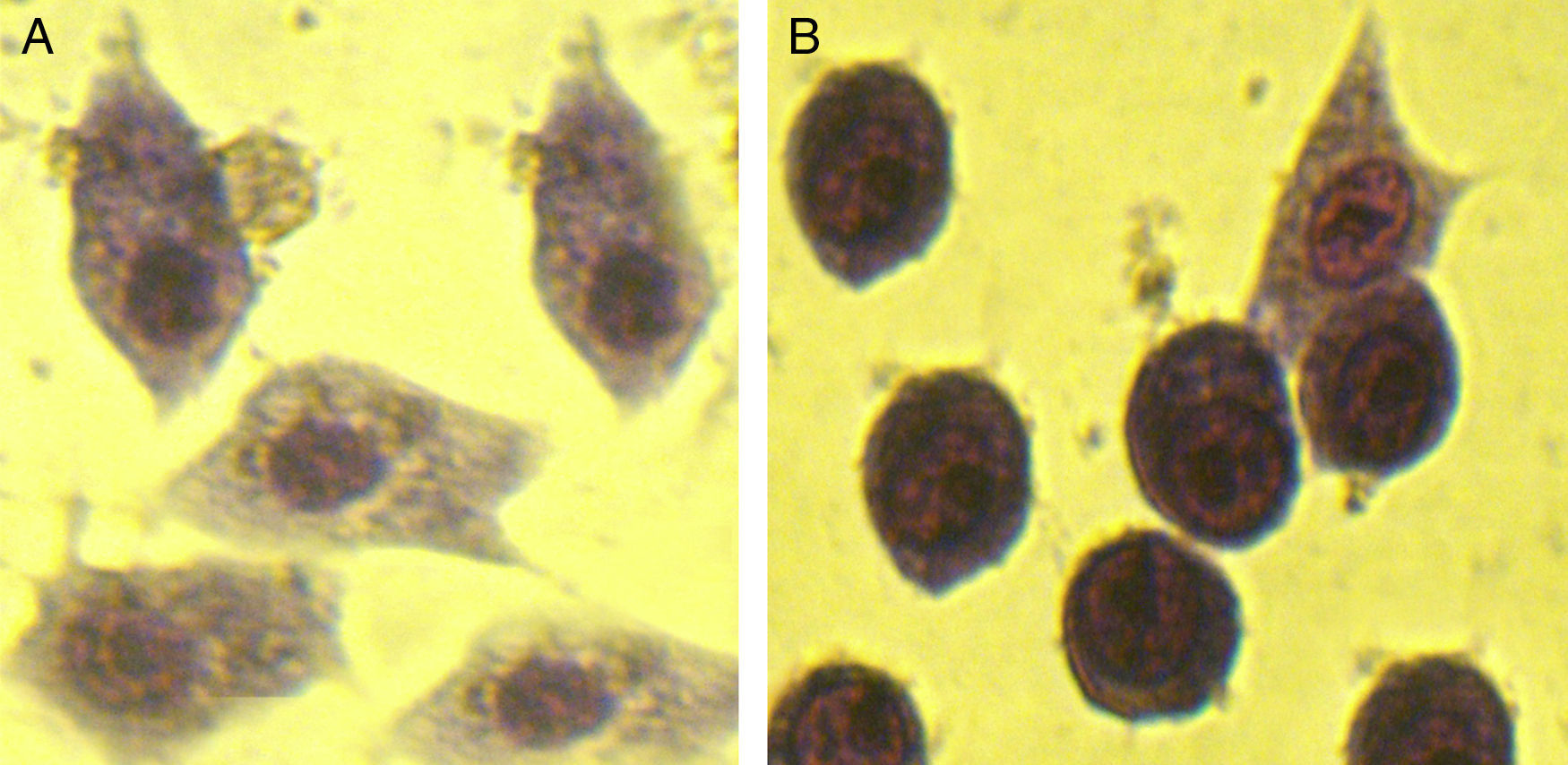
For cell rounding assay, Y-1 mouse adrenal cells (Pasteur Institute of Iran) were seeded in cell culture plates at a concentration of 5×104 cells per well and grown to confluence at 37°C/5% CO2 atmosphere. Y-1 cells were mixed with LT pretreated with serum samples and incubated under 5% CO2 incubator at 37°C for 10h. The morphological changes were observed under the compound microscope.
Statistical analysisAll statistical analyses were conducted using a SPSS 12.0 statistical program. Student's t-test was used to analyze the data for antibody responses between immunized and non-immunized groups; t-test was also used to evaluate the significance of differences in inhibition of ETEC binding to Caco-2 cells generated by test sera. A value of p<0.05 was considered statistically significant.
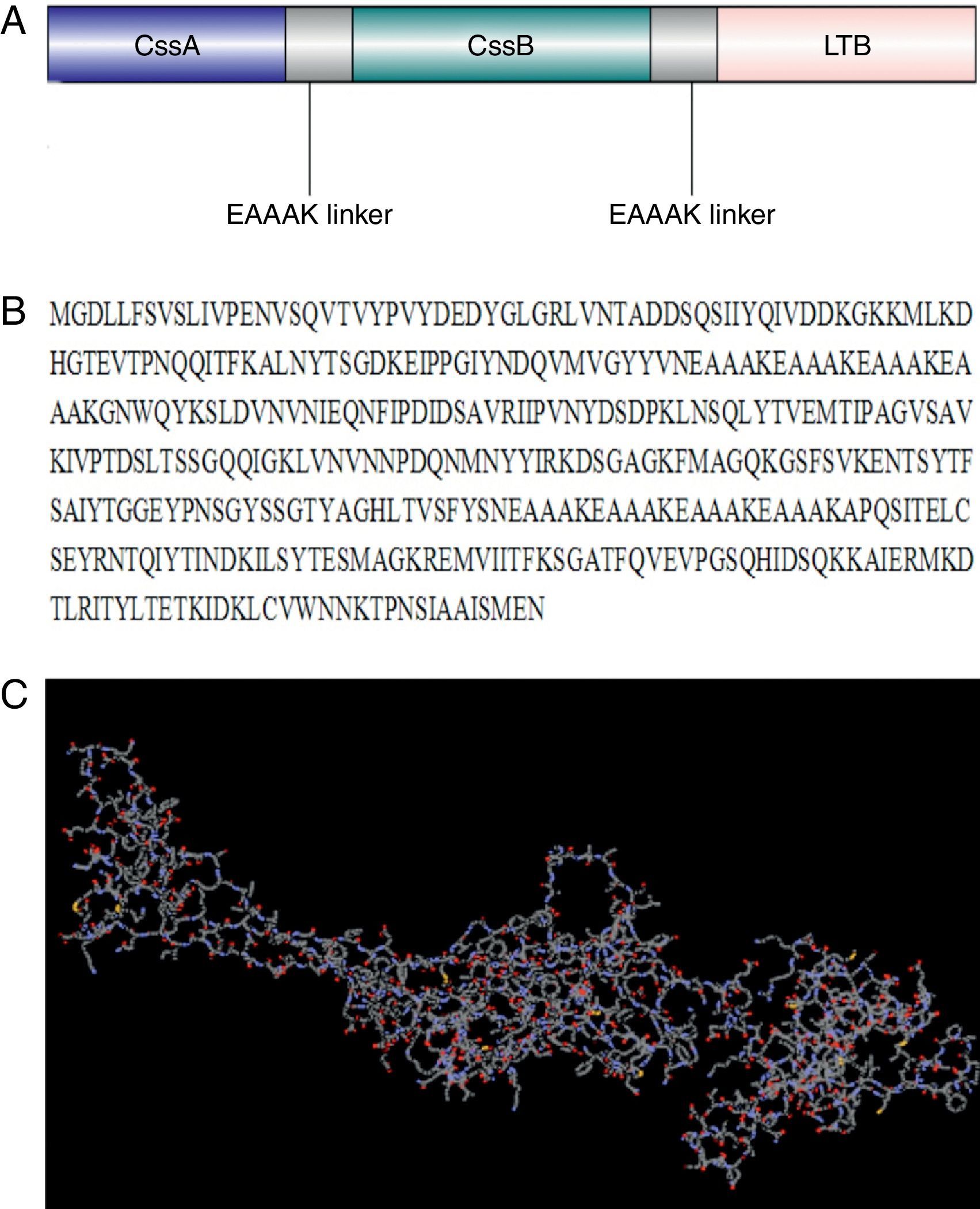
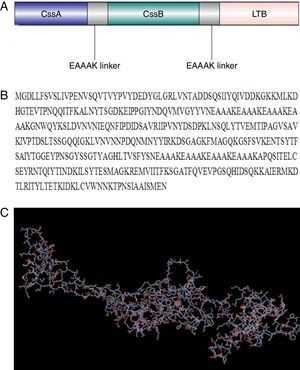
ResultsCodon optimization and tertiary structure predictionA chimeric trivalent protein fused together by (EAAAK)4 hydrophobic linkers in order to find the best epitope exposing chimeric antigen (Fig. 1A and B). Codon optimization was performed to improve the transcription efficiency and transcript stability. Percentage of codon having a frequency distribution of 91–100 in the native chimeric CS6 gene was 66%, which was significantly improved to 91% in the optimized gene sequence. The overall GC content, which is a measure of transcriptional and translational efficiency, was improved from 38.96% to 48.75% upon codon optimization. There were five negative cis elements in the native CS6 gene sequence, which were removed after optimization.
Design and modeled tertiary structure of chimeric protein: (A) schematic construction of recombinant chimeric protein containing CssB, CssA and LTB bound together by appropriate linkers, (B) amino acid sequences of chimeric protein and (C) modeled tertiary structure of chimeric protein by I-TASSER software. This model showed a protein with three main domains linked together with linkers.
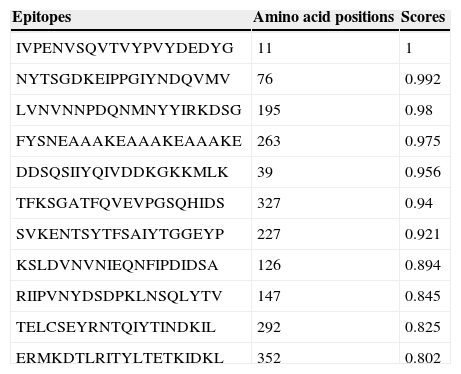
Ab initio modeling of the synthetic sequence was exploited to produce 3D models of the chimeric protein. The result of tertiary structure of the chimeric protein construction using I-TASSER showed a protein with three main domains linked together with linker (Fig. 1C). B-cell epitope was predicted and the epitopes having cutoff values >0.8 were selected (Table 1).
The predicted B-cell epitopes from full-length chimeric protein.
| Epitopes | Amino acid positions | Scores |
|---|---|---|
| IVPENVSQVTVYPVYDEDYG | 11 | 1 |
| NYTSGDKEIPPGIYNDQVMV | 76 | 0.992 |
| LVNVNNPDQNMNYYIRKDSG | 195 | 0.98 |
| FYSNEAAAKEAAAKEAAAKE | 263 | 0.975 |
| DDSQSIIYQIVDDKGKKMLK | 39 | 0.956 |
| TFKSGATFQVEVPGSQHIDS | 327 | 0.94 |
| SVKENTSYTFSAIYTGGEYP | 227 | 0.921 |
| KSLDVNVNIEQNFIPDIDSA | 126 | 0.894 |
| RIIPVNYDSDPKLNSQLYTV | 147 | 0.845 |
| TELCSEYRNTQIYTINDKIL | 292 | 0.825 |
| ERMKDTLRITYLTETKIDKL | 352 | 0.802 |
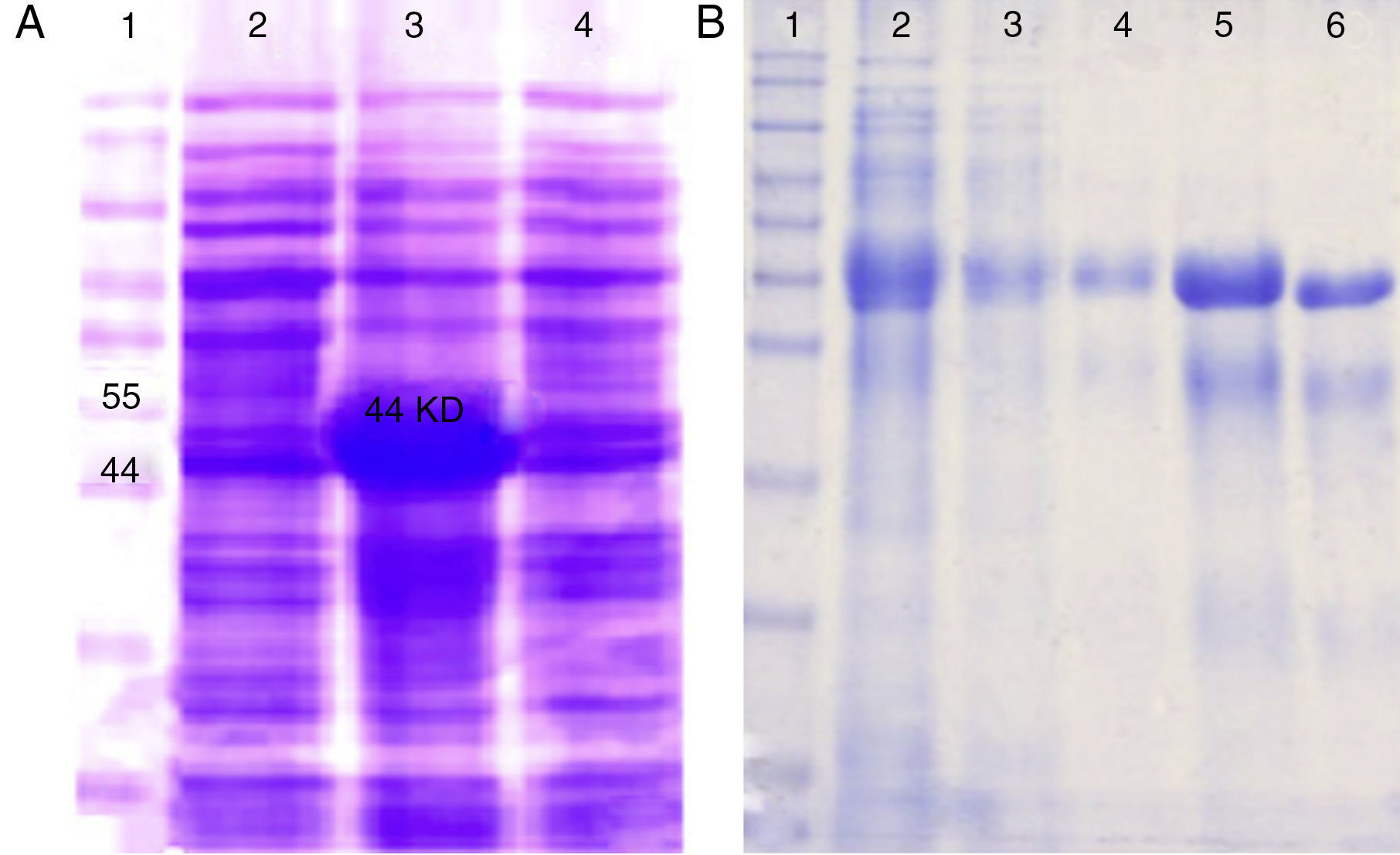
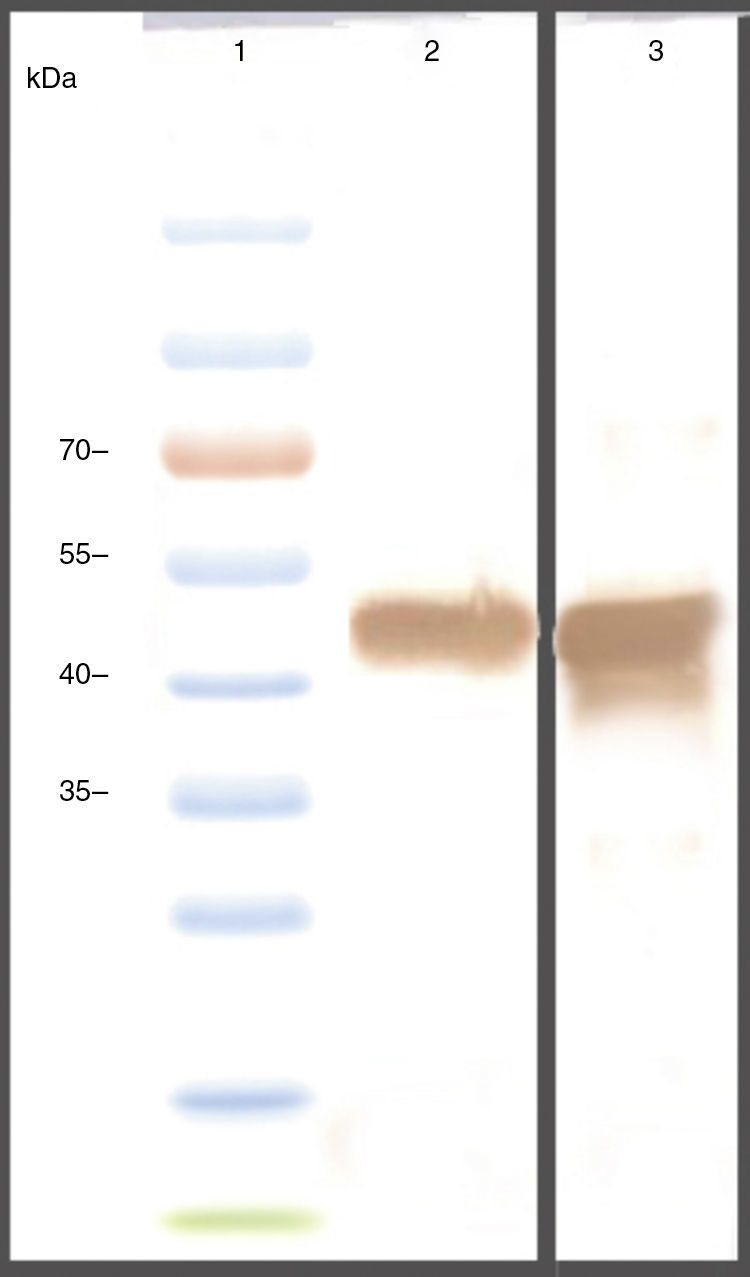
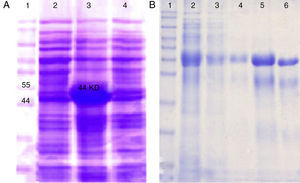
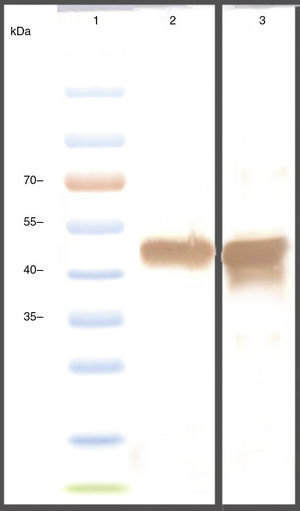
The genes were subcloned into pET28a and pET32a vectors respectively and over expressed in E. coli (BL21DE3) (Fig. 2A). The SDS-PAGE analysis showed the presence of a 47 KD recombinant chimeric protein. The purification of recombinant proteins was carried out under denaturation condition by Ni-NTA affinity (Fig. 2B). The expression of the recombinant protein was confirmed by the detection of the protein by Western blot using anti-His-tag and anti CTX antibodies (Fig. 3).
Expression and purification of recombinant chimeric protein: (A) SDS-PAGE analysis of the rCS6 expression after IPTG induction. Lane 1: protein weight marker. Lanes 2, 4: non induced cells. Lane 3: the induced bacteria. (B) Purification of rCS6 protein by Ni-NTA column. Lane 1: protein weight marker. Lane 2: expression of chimeric protein. Lane 3: flow-through. Lane 4: column washed with buffer D (pH=5.9). Lane 5: column washed with elution buffer (pH=4.5). Lane 6: column washed with mes Buffer.
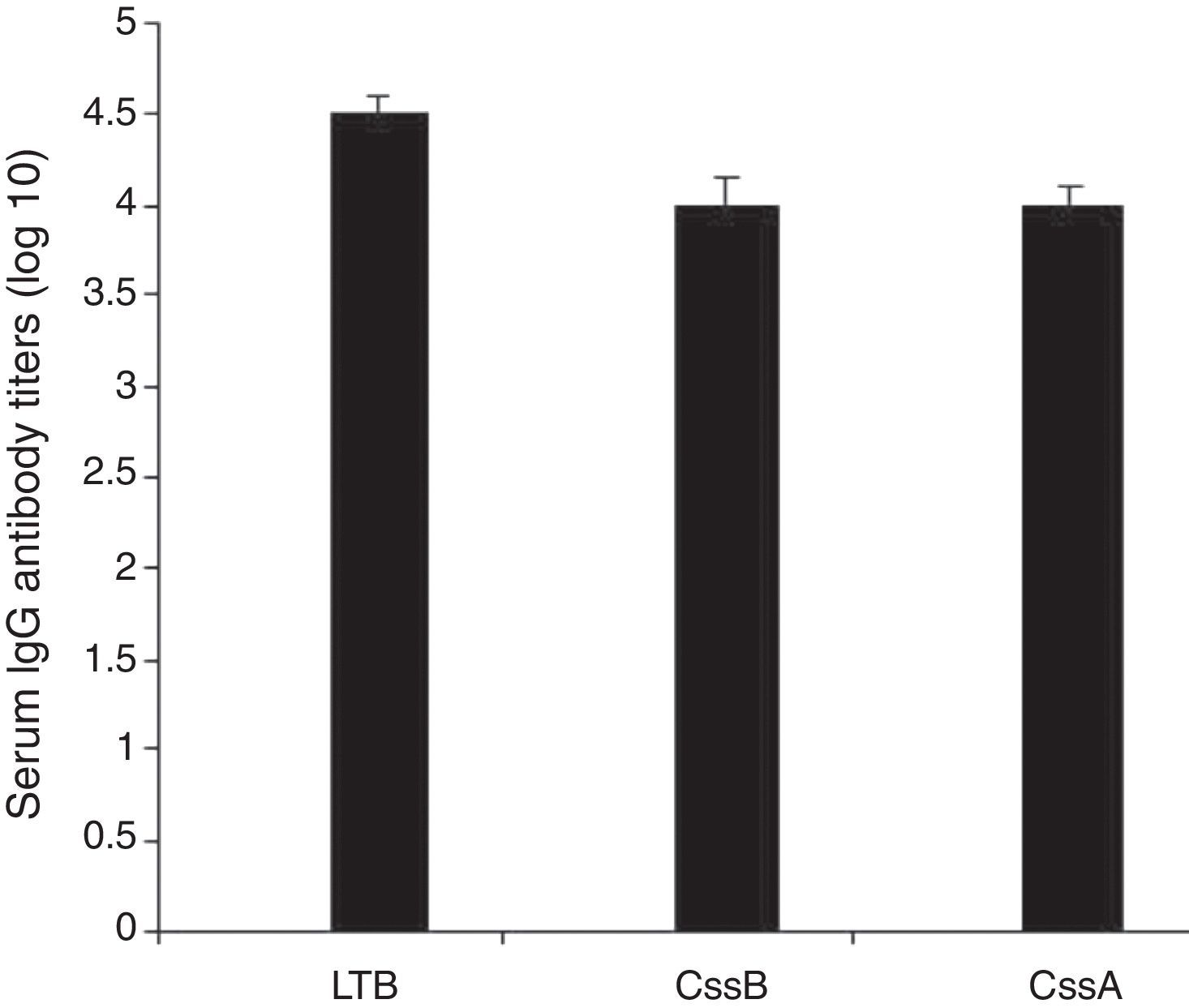
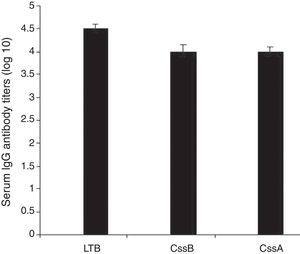
Animals were immunized with the recombinant chimeric protein. All immunized animals had anti-CssA, anti-CssB, and anti-LT IgG antibodies detected in serum (Fig. 4).
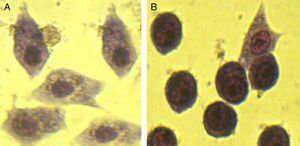
Inhibition of ETEC binding to Caco-2 cellsAssessment of Caco-2 cell monolayers revealed that ETEC cells pretreated with serum from non-immunized mice were distributed on the Caco-2 cells. However, pretreatment of the ETEC cells with immunized mice antisera considerably blocked their binding to Caco-2 cells. The immune sera could inhibit 68±3.5% bacterial adhesion onto the target cells. Comparison of pretreatment of the bacteria with non-immune antisera with that of immunized mice serum, showed a considerable reduction in their adhesion to Caco-2cells followed by pretreatment of the ETEC cells with immunized mice antisera.
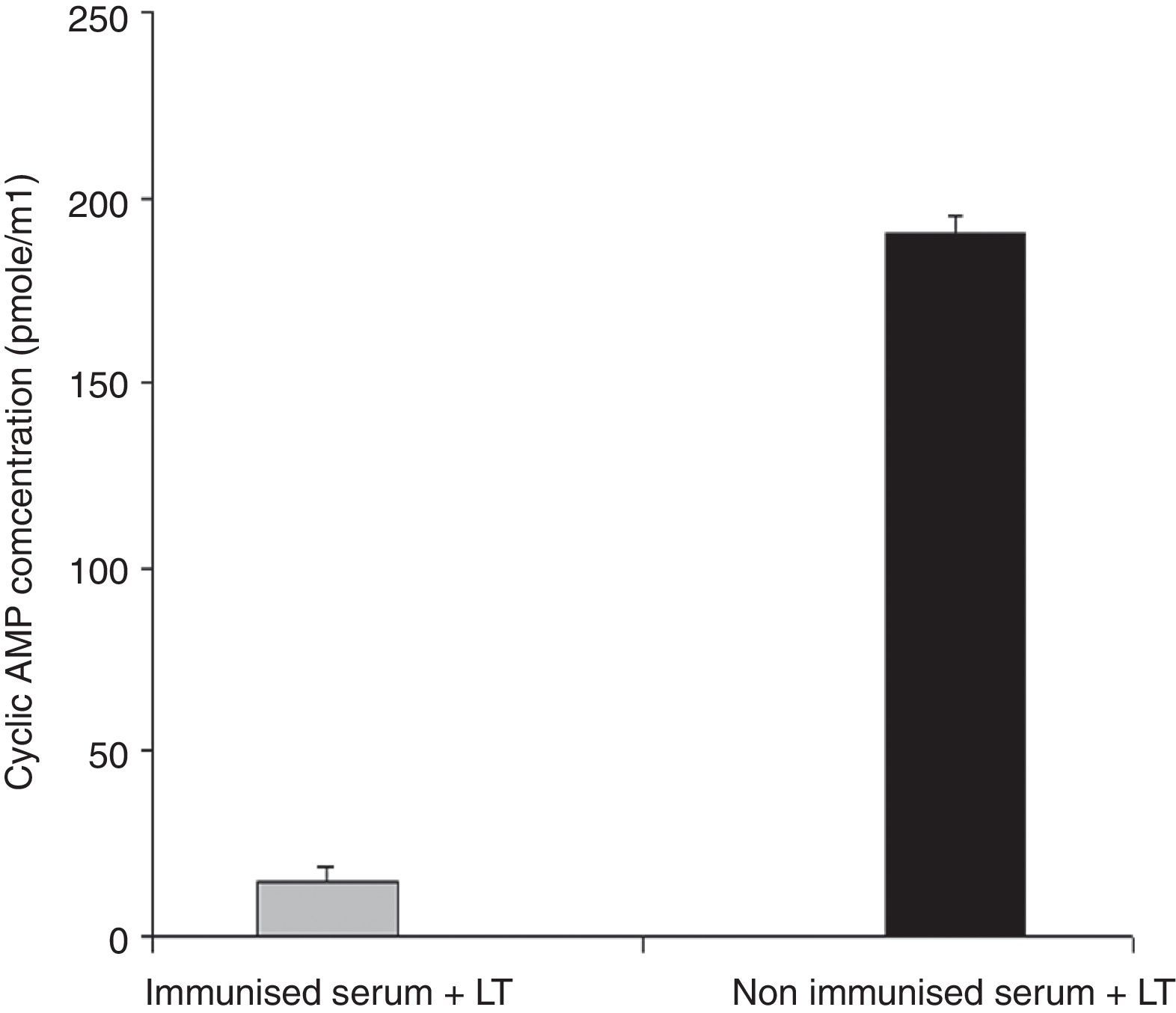
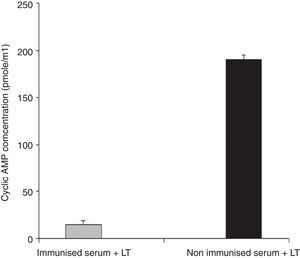
Anti-LT toxin antibody neutralization assayFor each ileal loop, the volume of secretion measured after excision was as follows: ETEC as a positive control: 2.59±0.3g/cm, ETEC+ antisera: 0.51±0.01g/cm, PBS: 0.31±0.12g/cm. The fluid accumulation was not observed in rabbit ileal loops 18h post-infection with ETEC treated with the immunized mice serum. In contrast, infection with standard ETEC was accompanied by an increase of fluid secretion. Data from the cAMP immunoassay kit showed that antibodies in serum and fecal samples of the immunized mice neutralized LT and prevented the toxin from stimulating intracellular cyclic AMP levels in CHO cells (Fig. 5). No morphological changes were observed in Y1 cells incubated with LT pretreated with anti-chimeric antibody, whereas normal serum had no inhibitory effects on rounding (Fig. 6).
Anti-LT antibodies neutralization assay with cAMP immunoassay. Serum samples from immunized or from the control mice were tested to neutralize LT toxin. Cyclic AMP concentrations of treated CHO cells were measured using a cAMP kit. Mean values are shown, and error bars represent standard deviations.
A vaccine against ETEC could have a significant impact on morbidity and mortality caused by diarrheal diseases in children living in developing countries.19 ETEC vaccine development has been based on two strategies of blocking adherence and/or toxin activity.5 A trivalent recombinant protein was designed consisting of CssB, CssA, LTB subunits.
The production of chimeras consisting of ETEC adhesion and enterotoxin B subunit may be capable of eliciting both antitoxin and anti-adhesion immune responses.16 Chimeric method reduces the need for producing three separate antigens, which would make it more cost-effective and would ease the manufacturing process.
In the present study LTB reported to be expressed by over half of LT expressing ETEC isolates was used, the presence of antibody against LTB can block the binding of the toxin to epithelial cells.14,20 LTB shows adjuvant effect that elicited strong humoral and cellular immune response against the vaccine components.15,21 On the other hand, although ST antigens are prevalent in ETEC isolates, they cannot be used as vaccine components for their poor immunogenicity.11 CssB have been reported as a key factor for binding to host cells7 and a hundred amino acids from the C-terminal region of CssA have been reported to interact with the host cell fibronectin.4 We combined three fragments with linkers (EAAAK)4. Chimeric proteins with a helical linker are thought to have a more elongated conformation compared to those with a flexible linker. (EAAAK)4 sequences were introduced between different domains for more flexibility and efficient separation. The successful experience of using (EAAAK)4 sequences in chimeric gene has paved the way for it to bring about rationally acceptable results.17 The recombinant protein was confirmed by western blot using anti-His-tag -conjugated HRP and anti-CTXB antiserum as LTB is structurally very similar to B subunit of cholera toxin (CTXB). The purified rCS6 was used for immunization of mice and rabbit. In order to achieve an optimal induction and powerful response, we applied booster doses at least three weeks after the first injection. The doses were gradually decreased to increase affinity and to fully activate the B-cell immune cascade. The ELISA test results indicated that the immunized animals produced higher titer of anti-chimer antibody than each individual gene (to each individual gene alone) and immunization with chimeric rCs6 strongly protected animals from ETEC challenges. Accordingly a significant difference in antibody titer was observed between sera of the immunize animals and control groups. The finding further indicates that B-cell epitopes of this chimeric protein might be located in three distinct parts, selected as the CssA, CssB, and LTB.
The adhesion inhibition capacity of the antibodies raised against the recombinant chimer protein assayed with Caco-2 cells revealed that pretreatment of ETEC with immunized mice antisera decreased their adhesion properties and blocked their binding to Caco-2 cells. Attachment to epithelial cell culture of ETEC was effectively reduced in immunized mice compared to the control. This is consistent with the reports indicating that CssA, CssB were necessary for attachment to intestinal cells.4,7
The ability of the antibodies against rCS6 to block fluid accumulation was tested in rabbit intestinal loops. The results indicate that rCS6 antibody is capable of binding to the toxin and neutralizing its effect. Vaccination with a recombinant chimeric trivalent immunogen could protect the host against ETEC. Pretreatment of the ETEC cells with immunized mice antisera remarkably decreased their adhesion properties and blocked their binding to Caco-2 cells.
Since mucosal immunity, especially the production of IgA antibodies, is thought to be important for blocking the attachment of E. coli to epithelial cells, the latter aspect, such as the application of the chimeric recombinant protein in oral administration, should be considered in further immunization studies. However, it has been demonstrated with Citrobacter rodentium that secretory IgA is not necessary for preventing bacterial colonization in mice using intimin as a vaccine antigen.22
In conclusion, our chimeric protein not only induced anti-toxins neutralizing antibodies, but also induced anti-adhesion antibodies for protecting against ETEC. The findings are in support of its use as an immunogen protein toward taking advantage of its components in vaccine strategies against ETEC.
Conflicts of interestThe authors declare no conflict of interests.
The authors wish to thank Shahed University for the sanction of grants to conduct the present study.