Global dissemination of mcr-like genes represents a serious threat to public health since it jeopardizes the effectiveness of colistin, an antibiotic used as a last-resort treatment against highly antibiotic-resistant bacteria. In 2017, a mcr-1-positive isolate of Escherichia coli was found in Chile for the first time. Herein we report the genetic features of this strain (UCO-457) by whole-genome sequencing (WGS) and conjugation experiments. The UCO-457 strain belonged to ST4204 and carried a 285 kb IncI2-type plasmid containing the mcr-1 gene. Moreover, this plasmid was transferred by conjugation to an E. coli J53 strain at high frequency. The isolate harbored the cma, iroN, and iss virulence genes and did carry resistance genes to trimethoprim/sulfamethoxazole and fluoroquinolones. Other antibiotic resistance determinants such as β-lactamases-encoding genes were not detected, making the isolate highly susceptible to these antibiotics. Our results revealed that such susceptible isolates could be acting as platforms to disseminate plasmid-mediated colistin resistance. Based on this evidence, we consider that mcr-like prevalence deserves urgent attention and should be examined not only in highly resistant bacteria but also in susceptible isolates.
Colistin represents the last resort to treat serious infections caused by highly resistant Gram-negative pathogens, such as extensively drug-resistant (XDR) Enterobacteriaceae and non-fermenting bacilli.1 In late 2015, Liu et al. found a plasmid-borne mcr-1 gene, which confers colistin resistance, in Escherichia coli and Klebsiella pneumoniae from diverse sources in China including food products, livestock, and humans.1,2 This was the first description of a colistin resistance trait harbored by a mobile genetic element, thus implicating the likely horizontal spread of colistin resistance. Indeed, mcr genes have since been described across the world from a myriad of sources including food products, animals, humans, and environmental samples.1 In South America (SA), mcr-1 has been identified in Enterobacteriaceae isolated from Argentina, Ecuador, Colombia, Bolivia, Venezuela, and Brazil. In addition, mcr-5 has been reported in Brazil.3,4 So far, no other mcr-like genes have been identified in SA.
It is crucial to track the presence and dissemination of mcr-like genes since decreasing the effectiveness of colistin leaves no therapeutic option to tackle highly resistant Gram-negative bacteria. Unfortunately, data concerning colistin-resistance and prevalence of mcr-like genes in SA, and Chile in particular, are scant. To date, Legarraga et al.5 published the first and only clinical description of mcr-1 in Chile, which was found in an E. coli isolate (UCO-457) recovered from a urinary infection in an outpatient of a hospital located in Santiago, Chile.5 The authors further described that this isolate was resistant to ciprofloxacin and trimethoprim/sulfamethoxazole, and susceptible to β-lactams, aminoglycosides and nitrofurantoin.
In order to achieve a fuller characterization of the first mcr-1 positive E. coli strain, we aimed to carry out whole-genome sequencing (WGS) of the UCO-457 isolate and study the transferability of mcr-1 by conjugation. Antimicrobial susceptibility profiles were determined by the disc diffusion method following the CLSI guidelines.6 The minimum inhibitory concentration (MIC) to colistin (CST) was determined by the broth dilution technique, and the results were interpreted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST; http://www.eucast.org/clinical_breakpoints) guidelines. WGS was performed using the Illumina MiSeq platform with a coverage of 30× with 2 × 250 bp paired-end reads. De novo assemblies were carried out by SPAdes v3.7 yielding 61 contigs (≥1000 bp). Species identification, sequence type (ST), serotype, Fim type, and plasmid replicon typing content were identified in silico using tools available at the Center for Genomic Epidemiology (CGE) server (http://www.genomicepidemiology.org/). Virulence genes were investigated using the virulence finder tool of the CGE server and the Pathosystems Resources Integration Center (PATRIC) toolkit (https://www.patricbrc.org/). Antibiotic-resistance genes (ARGs) were screened using ResFinder (http://cge.cbs.dtu.dk/services/ResFinder/) and the Comprehensive Antibiotic Resistance Database (CARD) (http://arpcard.mcmaster.ca).
The total genome length was 4,947,619 bp, with a N50 contig size of 210,597 bp and G + C content of 50.54%, consistent with other species in the Escherichia genus. This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession RXIT00000000. The version described in this manuscript is version RXIT00000000.1.
From the WGS data, we corroborated the identification of the isolate as E. coli using the SpeciesFinder tool on the CGE website. The isolate corresponded to ST4204 (CC10) according to the Warwick MLST scheme. Interestingly, mcr-1-positive isolates collected from urinary tract infections in China have also been associated to this ST.7,8 The plasmid containing the mcr gene in these isolates also carried the extended-spectrum β-lactamase gene blaCTX-M-55, in which both resistance genes were co-transferred by conjugation.7,8 The strain was negative for blaCTX-M, consistent with its observed susceptibility to β-lactams.5
Likewise, in silico typing revealed that UCO-457 corresponded to the serotype O6:H10 and belonged to the subclone fimH24. This serotype has been previously defined as pathogenic, since it typically produces toxins.9 However, we did not detect any toxin-related genes, although we did find diverse virulence genes such as cma (accession nº FJ664752), iroN (accession nº AF449498) and iss (accession nº DQ381420). The cma gene encodes a colicin M protein, which corresponds to a bacteriocin,10 while IroN corresponds to a enterobactin siderophore receptor protein, which is considered to be a urovirulence factor.11 Moreover, iss encodes a protein that increases the serum survival of E. coli, thus conferring resistance to the complement system.12
E. coli ST131 is a globally disseminated multidrug-resistant clone involved in urinary tract and bloodstream infections which possesses the type 1 fimbriae fimH30 allele.13 As described above, UCO-457 harbored the fimH24 subtype and thus is not related to the uropathogenic clone ST131. It is important to strengthen the national surveillance in Chile since there are not data about the fim subtypes circulating in the country. For instance, the fimH24 subtype, which is largely unknown in Chile, has been associated to national outbreaks in Denmark.14
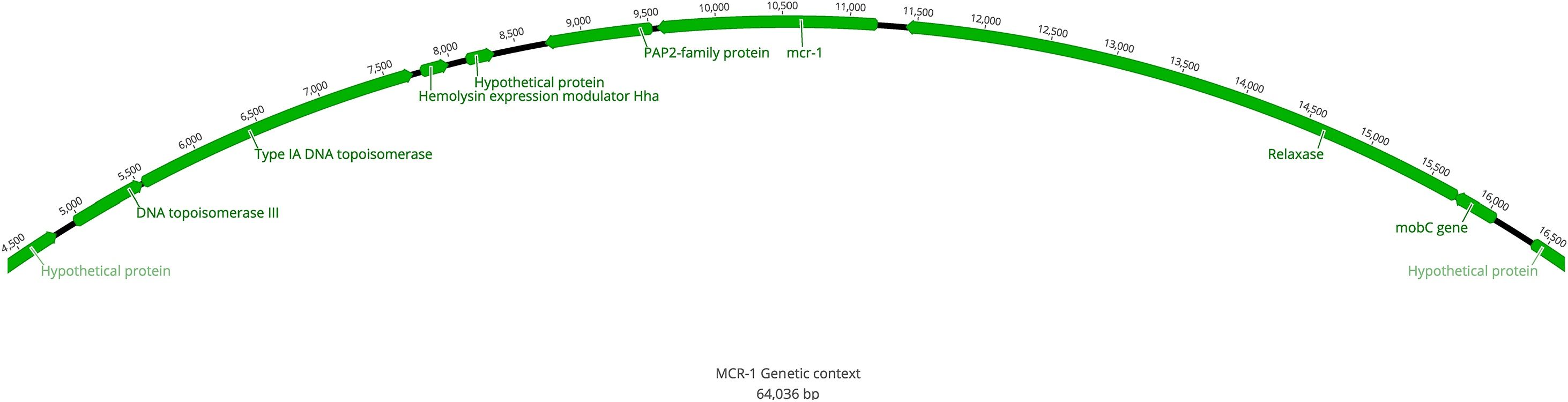
As expected, the WGS data analysis revealed the presence of the colistin-resistance gene mcr-1. Specifically, this gene was detected in a contig of 64,036 bp which did not contain any additional ARGs. Within the contig, mcr-1 was delimited upstream by a gene that encodes a PAP2-family protein and downstream by a relaxase-encoding gene (Fig. 1). This genetic environment differs from that commonly associated to mcr-1 in which the ISApl1-transposon is linked to this gene and mediates its dissemination.15 We detected the IncI2-replicon sequence in the same 64,036 bp contig with mcr-1, but did not detect any ISApI1 elements surrounding the mcr-1 gene (Fig. 1). This arrangement is reminiscent of the mcr-carrying E. coli from Argentina and Canada characterized by Tijet et al. where they found that the segment containing the mcr-1 gene had the pap2 and relaxase genes,16 as found in our isolate (Fig. 1). Moreover, some of the colistin-resistant E. coli isolates analyzed in their study also lacked the ISApI1 element. This could be explained by the mobilization of the IS resulting in the transfer of the mcr-1-pap2 arrangement to a conjugative plasmid with consequent loss of ISApI1,16 a process that could also have occurred in UCO-457.
Among Enterobacteriaceae, mcr-1 has been associated with many different incompatibility plasmid groups including IncI2, IncX4, IncHI1, IncHI2, IncFI, IncFII, IncP, IncI2-FIB, IncX1-X2, and IncX3-X4.17 Based on phylogenetic analysis, it has been suggested that IncI2 plasmids can migrate among different bacterial species with E. coli acting as a carrier of mcr-1 between them.18 Interestingly, the pap2-mcr-1 element has been previously detected in IncI2-plasmids.16 Although IncI2 plasmids are frequently associated with blaCTX-M-55 and blaKPC-3,18 these genes were not detected in UCO-457. These intriguing patterns warrant further study of the prevalence and potential role of this plasmid group in the dissemination of mcr-1.
In addition to mcr-1, the sul2 and dfrA14 genes were identified explaining the resistance phenotype to trimethoprim/sulfamethoxazole. We found that the IntI1 gene was in the same contig as dfrA14, suggesting this gene could be associated to a class 1 integron. This finding was confirmed by the NCBI Prokaryotic Genome Annotation Pipeline (PGAP),19 in which the dfrA14 gene was located immediately downstream the integrase gene (GenBank accession number RXIT01000051.1). We did not find any genes associated with the 3′ conserved region of these genetic elements, such as the qacEΔ1 and sul120 elements, indicating that some re-arrangements could be present in the structure of the detected integron.
The UCO-457 isolate had also been reported to be susceptible to amikacin and gentamicin and resistant to streptomycin. We detected the aph(3″)-Ib gene in the genome. This gene (also named as strA) mediates resistance specifically to streptomycin, explaining the observed phenotype.21 Finally, in addition to acquired resistance genes, we detected two-point mutations, S83L and D87N in gyrA, as well as a single point mutation S80I in parC, which are involved in resistance to ciprofloxacin.22
In complement to the WGS analysis, the plasmid content of UCO-457 was determined by the alkaline lysis method followed by pulsed-field gel electrophoresis (PFGE). Pure DNA plasmid extracts were used to examine whether mcr-1 was contained in this mobile element by PCR. Moreover, mcr-1 transferability was studied by conjugation using the E. coli J53 strain (sodium azide resistant and colistin susceptible) as the recipient strain. Susceptibilities to β-lactams, aminoglycosides, nitrofurantoin and CST in addition to their isogenicity and the presence of mcr-1 and IncI2-replicon were determined by the disk diffusion test, broth microdilution, ERIC-PCR and conventional PCR, respectively. From these studies, we detected a large plasmid of ca. 285 kb which contained the mcr-1 and the IncI2 sequence in UCO-457, which were also detected in the transconjugant E. coli J53-MCR1. Additionally, we determined that the plasmid transfer occurred with a high frequency of 2.8 × 10−4 transconjugants per recipient. It is important to note that the Illumina platform generates short reads, making it difficult to determine the entire sequence of plasmids 23. Due to the generation of these short reads, the use of Illumina to predict plasmids (i.e., by PlasmidFinder) is limited, being only possible to search for the incompatibility groups in the draft genome data generated from WGS.23 As a consequence, it is necessary to isolate and sequence the plasmid containing the mcr-1 in UCO-457 in order to determine its complete sequence.
MIC to CST of E. coli J53 varied from 2 µg/mL (J53) to 8 µg/mL (J53-MCR1) after the mating experiments, reaching the same value as the donor strain. Notably, the susceptibility pattern of J53-MCR1 to other antibiotics was not altered, demonstrating that only mcr-1 was transferred during this process. This finding confirms that the mcr-1 gene was the only antibiotic-resistance gene transferable at a high frequency. Similar results were obtained by Tijet et al., in which they described that colistin was the only antibiotic for which the resulting transformant was resistant after conjugation from mcr-1-carrying E. coli as a donor.16 As the UCO-457 isolate was recovered from a urinary tract infection from an outpatient, it is difficult to track the further spread of mcr-1 from this case. To the authors’ knowledge, there have been no additional reports of mcr-like genes in Chile to date.
In conclusion, we characterized the first mcr-1-positive E. coli isolated in Chile and found it to be part of the CC10 identified in China. The mcr-1 gene was detected in an IncI2-plasmid, which is known to be promiscuous. Therefore, further investigation is warranted in order to determine the prevalence and dissemination of this mcr-carrying plasmid. Finally, the isolate in which mcr-1 was detected was highly susceptible to other antibiotics, highlighting the potential role for such isolates to act as platforms for the dissemination of mcr genes.
Conflicts of interestThe authors declare no conflicts of interest.
Funding sourceAttraction and Insertion of Advanced Human Capital Program (PAI), CONICYT, Chile, under the project PAI79170082.
The authors want to thank to the National Commission for Scientific and Technological Research of Chile (CONICYT), for supporting this study. We thank Prof. Ellen Leffler for proofreading this manuscript. JRC–P thanks the support of Fondecyt regular (No 1190652).