Hepatitis E virus (HEV) can cause chronic infection with rapid progression to liver cirrhosis in immunocompromised patients. HEV seroprevalence in patients with Schistosoma mansoni in Brazil is unknown. We evaluated the prevalence of past or present HEV infection in schistosomiasis patients in Recife, Pernambuco, Brazil. A total of 80 patients with Schistosoma mansoni were consecutively enrolled in a cross-sectional study. Serum samples were tested for the presence of anti-HEV IgG antibodies by enzyme immunoassay (Wantai anti-HEV IgG, Beijing, China) and for the presence of HEV RNA using real time reverse transcriptase-polymerase chain reaction with primers targeting the HEV ORF2 and ORF3. Clinical and laboratory tests as well as abdominal ultrasound were performed at the same day of blood collection.
ResultsAnti-HEV IgG was positive in 18.8% (15/80) of patients with SM. None of the samples tested positive for anti-HEV IgM or HEV-RNA. Patients with anti-HEV IgG positive presented higher levels of alanine aminotranferase (p=0.048) and gama-glutamil transferase (p=0.022) when compared to patients without anti-HEV IgG antibodies.
ConclusionThis study demonstrates that the seroprevalence of HEV is high in patients with Schistosoma mansoni in Northeastern of Brazil. Past HEV infection is associated with higher frequency of liver enzymes abnormalities. HEV infection and its role on the severity of liver disease should be further investigated among patients with Schistosoma mansoni.
Hepatitis E virus (HEV) presents as large epidemics and sporadic cases in endemic areas, including genotype 1 in Asia and Africa, genotype 2 in Mexico and Africa, and genotype 4 in Asia. Sporadic cases of genotype 3 occur in Europe, Japan and the Americas. Genotypes 1 and 2 are restricted to primates and are transmitted predominantly by the fecal-oral route. Genotypes 3 and 4 infect numerous mammalian species and can be transmitted through the ingestion of raw or undercooked meat from infected animals.1
HEV infection usually presents as an acute self-limiting hepatitis, but in immunocompromised patients it can cause chronic infection with rapid progression to liver cirrhosis.2 Brazil has been classified as moderately endemic for HEV, with seroprevalence ranging from 1% to 4% in the general population and 15% in renal transplant recipients.3,4
Schistosoma mansoni (SM) is endemic in up to eight countries and islands in Latin America and the Caribbean, including Brazil. Up to 6 million people are infected, most of whom live in Northeastern Brazil and Venezuela, and 25 million are at risk for the infection.5 Schistosomiasis mansoni may progress to the most advanced form of disease, which is commonly observed in endemic areas. When this hepatosplenic form occurs in association with other hepatic disease, such as viral hepatitis, hepatic fibrosis can progress into cirrhosis within a few years.6
The seroprevalence of HEV in patients with SM in Brazil is unknown. The aim of this study was to evaluate the prevalence of past or present HEV infection in a sample of schistosomiasis patients in Recife, an endemic region of Northeastern, Brazil, and to associate the positivity to HEV infection to clinical and laboratory abnormalities.
Patients and methodsStudy areaRecife is the seventh largest metropolitan area in Brazil with approximately 3.9 million inhabitants, the second largest metropolitan area of the Northern/Northeastern Regions, and the capital and largest city of the state of Pernambuco.7 In addition, the hospital where the study was carried out is a reference center for patients with the most severe forms of schistosomiasis mansoni and receives patients from both Recife metropolitan region as well as from the endemic zone of Pernambuco State.8
Study designA cross-sectional study was carried out involving patients with SM who consecutively underwent an ultrasound exam over a nine-month period at the Division of Gastroenterology, of the Federal University of Pernambuco, Brazil. The diagnosis of SM was based on their clinical history of contact with contaminated water, positive parasitological stool examination for SM and/or reports of prior treatment for this parasite. In addition, to be included patients ought to have periportal fibrosis (PPF) on ultrasound evaluation of the liver.
Male and female patients aged 14 years or older with SM diagnosis were included. Those with the following criteria were excluded: presence of markers for hepatitis B or C (anti-HBc and anti-HCV); alcohol intake >210g/week; and ultrasound evidence of other liver disease, as expressed by the presence of steatosis or fine fibrosis diffused throughout the parenchyma.
During the study period 122 patients were evaluated; however, 42 patients (32.7%) were excluded due to co-infection with HBV and/or HCV, alcohol abuse, or other liver diseases as shown on liver ultrasound. Therefore, 80 patients were included in this study.
Ultrasound exams were performed by a single operator (ALCD) using the Siemens Acuson X 150® device with a 3.5MHz convex transducer for the evaluation of periportal fibrosis based on the Niamey classification, which has six pre-established patterns of fibrosis (PPF) intensity, ranging from Pattern A (normal) to Pattern F (very advanced fibrosis).9
According to the pattern of PPF by ultrasound, patients were divided into three groups: (1) mild group PPF: A+B; (2) moderate PPF: C+D; and (3) advanced PPF E+F.
Sample and data collectionSerum samples from these patients were collected for laboratory analysis. These included alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (AP), gamma-glutamyl transpeptidase (GGT), total protein and albumin, total bilirubin, hemoglobin, leukocyte, and platelet count.
Normal levels of liver enzymes were ALT≤31UI/L; AST≤31UI/L, AP≤105UI/L; and GGT≤41UI/L.
Anti-HEV antibodies detectionThe presence of anti-HEV IgG antibodies was investigated through enzyme immunoassay using the WANTAI HEV-IgG ELISA kit (Beijing Wantai Biological Pharmacy Enterprise, Beijing, China), strictly according to the manufacturer's recommendations. Specimens with positive results were tested for anti-HEV IgM antibodies using a specific kit from the same manufacturer.
RNA extraction and quantitative RT-PCRHEV RNA was extracted from fecal samples using QIAamp viral RNA mini kit (QIAGEN, Hilden, Germany), strictly according to the manufacturer's instructions.
Quantitative RT-PCR was performed according to a modified 1-step triplex real time protocol previously described10 with a set of primers and probe targeting a highly conserved 70nt long sequence within overlapping parts of ORF2 and ORF3,11 and another set specific for a 113nt sequence of ORF2.12 A third set of primers and probe targeting the human RNAseP gene was used as endogenous internal amplification control to certify specimen quality and RNA extraction.13
A plasmid clone from a Brazilian human HEV strain previously characterized (GenBank accession number KF1528840)14 was constructed with TOPO® TA cloning® kit (Invitrogen, Carlsbad, CA, USA) and the primers described. Plasmid DNA was purified using QIAprep spin miniprep kit (QIAgen, Hilden, Germany), linearized and quantified with the Nanodrop ND-1000 instrument (Wilmington, DE, USA) following transcription to RNA with T7 RNA polymerase (Promega, Madison, WI, USA). Standard curves were generated using 100 to 1010 copies of plasmid RNA. HEV viral loads were determined based on the standard curves. While qualitative real time RT-PCR showed a limit of detection of 5 copies of RNA per reaction, the linearity of quantitation was set as 50 copies of RNA per reaction (lowest reproducible viral load). All screening reactions were run in duplicates, with positive results confirmed in a separate confirmatory reaction.
Statistical analysisAll data were stored and analyzed using SPSS version 11.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics consisted of the characterization of the studied population (demographic, clinical and laboratory characteristics) and anti-HEV IgG seroprevalence through the respective rates or mean/median and standard deviation (SD) for continuous variables. The univariate analysis consisted of Pearson's Chi-square test to compare categorical values. For continuous variables, Student's t-test was used to compare means of normally distributed variables, while non-normally distributed variables were subjected to Mann–Whitney U test for comparison of medians. Statistical significance level was p<0.05. All reported values are two-tailed.
Ethical aspectsThe study protocol was approved by the Institutional Ethics Committee (162/09 and 86730/14) and all participants signed an informed consent form.
ResultsStudy subjects were 14–78 years old, median age 51 years old (mean±SD: 50.2±13.7); 52 (65%) patients were female. Schistosomiasis was clinically classified as mild (2/80; 2.5%), moderate (52/80; 65%), or severe (26/80; 32.5%), based on staging of periportal fibrosis, according to Niamey classification.15
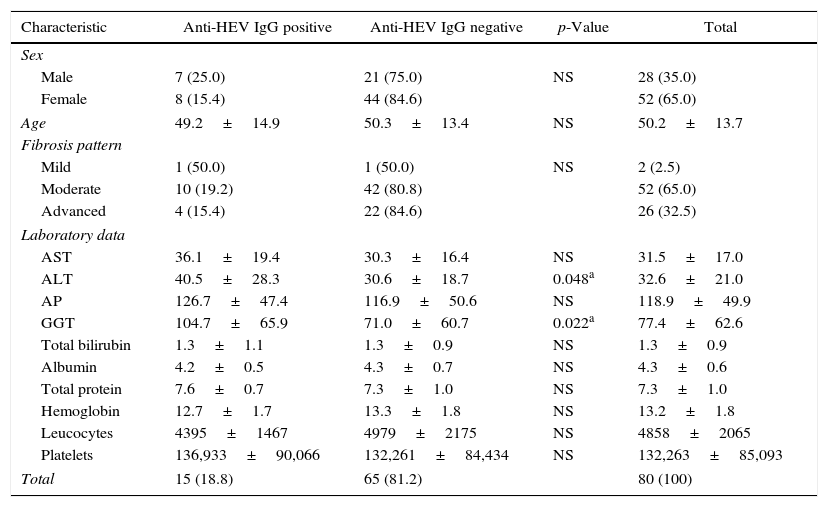
Anti-HEV IgG was positive in 15 of the 80 schistosomiasis patients (18.8%). None of the samples tested positive for anti-HEV IgM or HEV-RNA. Demographic, clinical and laboratory data are shown in Table 1.
Demographic, clinical and laboratory characteristics of schistosomiasis patients according to anti-HEV IgG status, Northeastern Brazil.
| Characteristic | Anti-HEV IgG positive | Anti-HEV IgG negative | p-Value | Total |
|---|---|---|---|---|
| Sex | ||||
| Male | 7 (25.0) | 21 (75.0) | NS | 28 (35.0) |
| Female | 8 (15.4) | 44 (84.6) | 52 (65.0) | |
| Age | 49.2±14.9 | 50.3±13.4 | NS | 50.2±13.7 |
| Fibrosis pattern | ||||
| Mild | 1 (50.0) | 1 (50.0) | NS | 2 (2.5) |
| Moderate | 10 (19.2) | 42 (80.8) | 52 (65.0) | |
| Advanced | 4 (15.4) | 22 (84.6) | 26 (32.5) | |
| Laboratory data | ||||
| AST | 36.1±19.4 | 30.3±16.4 | NS | 31.5±17.0 |
| ALT | 40.5±28.3 | 30.6±18.7 | 0.048a | 32.6±21.0 |
| AP | 126.7±47.4 | 116.9±50.6 | NS | 118.9±49.9 |
| GGT | 104.7±65.9 | 71.0±60.7 | 0.022a | 77.4±62.6 |
| Total bilirubin | 1.3±1.1 | 1.3±0.9 | NS | 1.3±0.9 |
| Albumin | 4.2±0.5 | 4.3±0.7 | NS | 4.3±0.6 |
| Total protein | 7.6±0.7 | 7.3±1.0 | NS | 7.3±1.0 |
| Hemoglobin | 12.7±1.7 | 13.3±1.8 | NS | 13.2±1.8 |
| Leucocytes | 4395±1467 | 4979±2175 | NS | 4858±2065 |
| Platelets | 136,933±90,066 | 132,261±84,434 | NS | 132,263±85,093 |
| Total | 15 (18.8) | 65 (81.2) | 80 (100) | |
Results are presented as number and percentage for categorical variables and as mean value and standard deviation for continuous variables. Conventional symbols used: – p>0.050. Note: Student's t-test was used for means comparison of normally distributed variables while Mann–Whitney U test was used for medians comparison of non-normally distributed variables. Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; AP, alkaline phosphatase; GGT, gama-glutamyl transpeptidase.
Patients with anti-HEV IgG antibodies showed significant increase in ALT, and GGT levels, when compared to patients with no evidence of HEV infection. Other laboratory parameters as well as the grade of liver fibrosis determined by ultrasound analysis showed no differences between the two groups.
DiscussionThe present data demonstrate that severe cases of SM continue to take place among outpatients in Northeastern, Brazil. Close to one-third (32.5%) of the patients exhibited advanced patterns (E and F) of periportal fibrosis. These patients with more advanced fibrosis and with low complete blood count presented with the hepatosplenic form of disease.16 Additionally, they showed higher levels of portal pressure and consequently a greater risk of digestive bleeding, requiring follow up in reference centers.9,17
In studies from 1997 to 2006, anti-HEV IgG prevalence varied from 2.0% to 4.3% in blood donors from Southeast and Northeast Brazil, and from 0% to 2.4% in individuals from rural and urban areas and in those living in low socioeconomic communities.18 A study on 699 patients of low-socioeconomic status living in Rio de Janeiro in 2002 reported a 2.4% seroprevalence.19
HEV seroprevalence rates appear to have increased in recent years.20 Indeed, in more recent studies, the prevalence of HEV infection varied from 5.1% in recyclable waste pickers from Central Brazil,21 10% in blood donors from the Southern part of the country,22 and 12.9% in rural Amazonia.23
In the present study we found a prevalence of 18.8% of anti-HEV IgG among a sample of schistosomiasis patients. Such prevalence is higher than that observed in immunocompetent populations in the country.18–23 There is only one study from Brazil that included, among other groups, patients with SM. In this study, 10% (3/30) of patients with SM were HEV IgG positive, also pointing to a high prevalence of HEV in this specific group of patients.24 Other studies with Egyptian patients have also demonstrated a higher prevalence of HEV infection in schistosomiasis patients.25–27 The higher prevalence of HEV infection in the schistosomiasis patients evaluated in the present study could be explained by the low socioeconomic conditions of these patients, which could have favored acquisition of both infections. Moreover, schistosomiasis may play a role in virus infection by altering host immune response, favoring the acquisition of infection. In fact, many studies demonstrate that the chronic phase of schistosomiasis infection is characterized by a state of immune hyporesponsiveness.28 Immunocompromised patients are known to be more susceptible to hepatitis E infection and even to chronic hepatitis E with rapidly progressive cirrhosis, a condition that was not detected in the present study. Fulminant hepatitis and higher mortality are also seen in individuals with underlying chronic liver disease.1,2 In developed countries, smaller studies have shown a poor prognosis for patients with underlying chronic liver disease, but it is not clear how frequently it occurs, as such patients are not routinely tested for evidence of HEV infection.1
In the present study, schistosomiasis patients with anti-HEV IgG antibodies showed significantly higher levels of ALT and GGT, when compared to patients with no evidence of past HEV infection. Although schistosomiasis per se can be associated with abnormal liver tests, mainly GGT,29,30 in the present study HEV infection seems to have contributed to the finding of more elevated levels of the liver enzymes. Accordingly, some studies have reported that anti-HEV IgG positive patients have higher levels of liver enzymes, when compared to non-infected patients.31,32 In the study by Hassing et al.,32 HIV-infected patients with anti-HEV IgG antibodies showed higher levels of ALT, when compared to anti-HEV IgG negative patients, and this elevation was not due to acute or chronic infection, since all patients were anti-HEV IgM and HEV-RNA negative, like in the present study. The finding of elevated ALT and GGT in patients with past HEV-infection, with or without underlying liver disease, merits further investigation in order to understand the mechanisms involved in these abnormalities.
In conclusion, this study demonstrates a higher seroprevalence of past HEV-infection in patients with SM from Recife, Northeastern Brazil. Also, schistosomiasis patients with evidence of past HEV-infection have higher levels of ALT and GGT, suggesting an interaction between these two liver diseases. Further studies are necessary to evaluate the epidemiology of HEV infection in SM patients and its impact on the severity of the disease.
FundingThis work was partially supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo [grant numbers 2012/22925-3, 2013/03701-0].
Conflicts of interestThe authors declare no conflicts of interest.