The aim of this study was to determine risk factors for acquiring carbapenem-resistant Pseudomonas aeruginosa bacteremia (CR-PA) and factors associated with in-hospital mortality.
MethodsSeventy-seven cases of bacteremia caused by P. aeruginosa were evaluated in a hospital with high incidence of CR-PA. Clinical and laboratorial factors, and previous use of antibiotics were also evaluated. In one analysis, CR-PA and carbapenem-susceptible P. aeruginosa (CS-PA) bacteremia were compared. A second analysis compared patients who died with survivors.
ResultsAmong 77 P. aeruginosa bacteremia, 29 were caused by CR-PA. Admission to the intensive care unit, higher number of total leukocytes, and previous use of carbapenem were statistically associated with CR-PA. In the multivariate analysis, only previous use of carbapenem (including ertapenem) turned out to be a risk factor for CR-PA (p=0.014). The 30-day mortality of patients with P. aeruginosa bloodstream infection was 44.8% for CS-PA and 54.2% for patients with CR-PA (p=0.288). Chronic renal failure, admission to the intensive care unit, mechanical ventilation, and central venous catheter were risk factors for mortality. Incorrect treatment increased mortality of patients with bacteremia caused by CS-PA, but not for CR-SA. The odd ratio of mortality associated with incorrect therapy in patients with CS-PA was 3.30 (1.01–10.82; p=0.043). The mortality of patients with bacteremia caused by CR-PA was unexpectedly similar regardless of antimicrobial treatment adequacy.
ConclusionAppropriate treatment for CS-PA bacteremia initiated within the first 24hours was associated with lower mortality, but this cannot be extrapolated for CR-PA.
Despite the wide distribution of P. aeruginosa in the environment, this microorganism rarely colonizes humans.1 However, the chance of colonization increases significantly in hospitalized patients.2 More than 70% of Pseudomonas infections occur as nosocomial or healthcare-associated infections.3 In some hospitals, P. aeruginosa can be the first agent of infection, mainly in respiratory and urinary tract infections.4 Bloodstream infections are mainly caused by Gram-positive cocci, although this rule cannot be expanded to developing countries, where environmental conditions favor Gram-negative bacilli infections.5 Some risk factors for Pseudomonas bacteremia have been described as increased age, hemodialysis, solid organ transplant, neoplasms, heart disease, diabetes mellitus, and chronic obstructive airway disease.6 However, these factors are unalterable, and efforts should focus on appropriate antibiotic therapy and prevention.
Resistance of P. aeruginosa against antipseudomonal drugs has increased due to different trends in several regions of the world. The choice of an ideal empiric antibiotic against a possible infection caused by P. aeruginosa has been a challenge. The mechanisms of resistance are complex, involving acquisition genes (mainly against beta-lactams and aminoglycosides) and chromosomal genes (against fluorquinolones). Carbapenem has been one of the most important classes of antibiotics used in the empirical treatment of nosocomial infections, mainly in severe cases. However, resistance of P. aeruginosa to imipenem and meropenem has reached more than 70% in some hospitals in Brazil.7,8 The main mechanisms of resistance to carbapenem are the loss of the OprDm efflux bomb and production of metallo-beta-lactamases, the latter may correspond up to 30% in a previous study in the South of Brazil.9–11
The multidrug-resistant P. aeruginosa is associated with increased mortality and costs due to prolonged hospitalization, need of surgery, and prolonged treatment with antibiotics.12 Considering the endemic condition of Pseudomonas infection in hospitals, the question is whether there is a certain risk group for multi-drug resistant (MDR) Pseudomonas infection, or are all the patients under such risk.
Considering the current scenario, a case-control study was performed to determine the risk factors associated with bacteremia caused by carbapenem-resistant P. aeruginosa (CR-PA), using as a control patients with carbapenem-susceptible strains of P. aeruginosa (CS-PA). Factors associated with mortality and other outcomes, especially the choice of antibiotic treatment for CR-PA, were also evaluated.
Patients and methodsPatientsA case control study was carried out at the Hospital Universitário Evangélico de Curitiba. This center is a 660-bed tertiary-care hospital in Curitiba, a city located in Southern Brazil. It is a reference center for trauma, burns, and renal transplantation with 60 intensive care beds.
All the patients older than 18 years with bacteremia caused by P. aeruginosa from February, 2006 to January, 2009 were included. Patients with more than one episode of Pseudomonas bacteremia were included once. Patients with bacteremia caused by other microorganism before P. aeruginosa were excluded.
Microbiological definitionCultures were collected according to the standard protocol used in the hospital and were processed using the BACT/Alert® (bioMérieux–Durham, USA). P. aeruginosa was identified using biochemical analysis.13 Susceptibility testing was performed by the disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) guidelines.14
Clinical findingsThe following variables were evaluated for each patient: gender; age; previous hospital admission within the last 90 days; admission to the intensive care unit (ICU); length of hospitalization before bacteremia; use of mechanical ventilation, central venous line, urinary catheter and surgery during the current hospitalization; underlying conditions such as diabetes mellitus, chronic renal failure, heart failure, and cancer; trauma and previous antibiotic use during current hospitalization; previous colonization by P. aeruginosa. The following laboratory parameters were evaluated on the day of diagnosis: hemoglobin, leukocyte, platelet counts, and creatinine.
Thirty-day and in-hospital mortality were registered. Antibiotic treatment was classified as correct or incorrect. Treatment of each patient was considered correct if the P. aeruginosa strain was susceptible to the antibiotic used/started in less than 24hours after blood collection.
Analysis of dataPatients with CR-PA bacteremia were compared with patients with CS-PA bacteremia to determine factors associated with carbapenem resistance. A second analysis was performed comparing patients who died during hospitalization with those who survived. Continuous data were expressed as mean±standard deviation (SD) or median with ranges. Frequencies were expressed as percentages. Medians were compared with the non-parametric test Kruskal-Wallis. Dichotomous variables were compared using the chi-square (χ2) test, and the Mann Whitney test was used for continuous variables. Significance level was set at 0.05. Variables in which p<0.10 in the univariate analysis were included in the multivariate analysis. Multivariate analysis was performed using a forward factorial binary logistic regression model. Odds ratios (OR) with 95% confidence intervals (95% CI) were calculated for each variable. Variables in which 95% CI did not include 1.0 were maintained in the final model.
Kaplan-Meier survival estimates were calculated to evaluate the role of correct treatment in the outcome of bacteremia caused by CR-PA, and the difference was assessed using the log-rank test. Significance was determined when the p-value was lower than 0.05.
All data were recorded using the software Excel (Microsoft–New York, USA) and analyzed with the free software R, version 2.11 (The R Foundation for Statistical Computing). Kaplan-Meier survival estimates were determined with GraphPad Prism 4.0 (GraphPad–La Jolla, USA).
ResultsA total of 77 patients were included in this study. Twenty-nine patients presented CR-PA bacteremia and 48 patients had CS-PA bacteremia. The median age was 49 years-old in the CR-PA group, and 46.5 in the CS-PA group, with no significant difference, using parametric (for mean) or non-parametric (for median) tests.
The duration of hospitalization before bacteremia was not different between the groups, although it had a tendency to be prolonged in the CR-PA group, with a median of 13.5 days in the CS-PA group and 20.0 days in the CR-PA group. The time of hospitalization was also not different between groups.
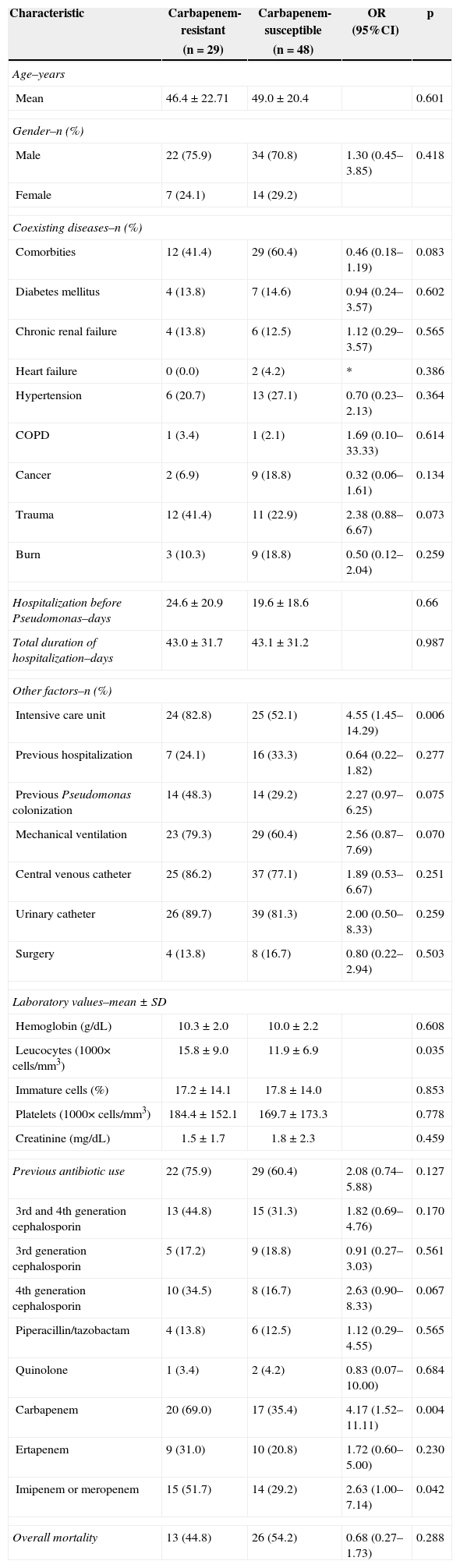
All the clinical and laboratorial data are detailed in the Table 1. Even though several variables were compared, admission at the intensive care unit, higher number of total leukocytes, and previous use of carbapenem were statistically significant. In the multivariate analysis only previous use of carbapenem (including ertapenem) remained as an independent risk factor for CR-PA (p=0.014).
Risk factors for carbapenem-resistant Pseudomonas aeruginosa bacteremia. Risk factors were compared with susceptible strains. Values are pictured as absolute numbers followed by percentage.
| Characteristic | Carbapenem-resistant | Carbapenem-susceptible | OR (95%CI) | p |
|---|---|---|---|---|
| (n=29) | (n=48) | |||
| Age–years | ||||
| Mean | 46.4±22.71 | 49.0±20.4 | 0.601 | |
| Gender–n (%) | ||||
| Male | 22 (75.9) | 34 (70.8) | 1.30 (0.45–3.85) | 0.418 |
| Female | 7 (24.1) | 14 (29.2) | ||
| Coexisting diseases–n (%) | ||||
| Comorbities | 12 (41.4) | 29 (60.4) | 0.46 (0.18–1.19) | 0.083 |
| Diabetes mellitus | 4 (13.8) | 7 (14.6) | 0.94 (0.24–3.57) | 0.602 |
| Chronic renal failure | 4 (13.8) | 6 (12.5) | 1.12 (0.29–3.57) | 0.565 |
| Heart failure | 0 (0.0) | 2 (4.2) | * | 0.386 |
| Hypertension | 6 (20.7) | 13 (27.1) | 0.70 (0.23–2.13) | 0.364 |
| COPD | 1 (3.4) | 1 (2.1) | 1.69 (0.10–33.33) | 0.614 |
| Cancer | 2 (6.9) | 9 (18.8) | 0.32 (0.06–1.61) | 0.134 |
| Trauma | 12 (41.4) | 11 (22.9) | 2.38 (0.88–6.67) | 0.073 |
| Burn | 3 (10.3) | 9 (18.8) | 0.50 (0.12–2.04) | 0.259 |
| Hospitalization before Pseudomonas–days | 24.6±20.9 | 19.6±18.6 | 0.66 | |
| Total duration of hospitalization–days | 43.0±31.7 | 43.1±31.2 | 0.987 | |
| Other factors–n (%) | ||||
| Intensive care unit | 24 (82.8) | 25 (52.1) | 4.55 (1.45–14.29) | 0.006 |
| Previous hospitalization | 7 (24.1) | 16 (33.3) | 0.64 (0.22–1.82) | 0.277 |
| Previous Pseudomonas colonization | 14 (48.3) | 14 (29.2) | 2.27 (0.97–6.25) | 0.075 |
| Mechanical ventilation | 23 (79.3) | 29 (60.4) | 2.56 (0.87–7.69) | 0.070 |
| Central venous catheter | 25 (86.2) | 37 (77.1) | 1.89 (0.53–6.67) | 0.251 |
| Urinary catheter | 26 (89.7) | 39 (81.3) | 2.00 (0.50–8.33) | 0.259 |
| Surgery | 4 (13.8) | 8 (16.7) | 0.80 (0.22–2.94) | 0.503 |
| Laboratory values–mean±SD | ||||
| Hemoglobin (g/dL) | 10.3±2.0 | 10.0±2.2 | 0.608 | |
| Leucocytes (1000× cells/mm3) | 15.8±9.0 | 11.9±6.9 | 0.035 | |
| Immature cells (%) | 17.2±14.1 | 17.8±14.0 | 0.853 | |
| Platelets (1000× cells/mm3) | 184.4±152.1 | 169.7±173.3 | 0.778 | |
| Creatinine (mg/dL) | 1.5±1.7 | 1.8±2.3 | 0.459 | |
| Previous antibiotic use | 22 (75.9) | 29 (60.4) | 2.08 (0.74–5.88) | 0.127 |
| 3rd and 4th generation cephalosporin | 13 (44.8) | 15 (31.3) | 1.82 (0.69–4.76) | 0.170 |
| 3rd generation cephalosporin | 5 (17.2) | 9 (18.8) | 0.91 (0.27–3.03) | 0.561 |
| 4th generation cephalosporin | 10 (34.5) | 8 (16.7) | 2.63 (0.90–8.33) | 0.067 |
| Piperacillin/tazobactam | 4 (13.8) | 6 (12.5) | 1.12 (0.29–4.55) | 0.565 |
| Quinolone | 1 (3.4) | 2 (4.2) | 0.83 (0.07–10.00) | 0.684 |
| Carbapenem | 20 (69.0) | 17 (35.4) | 4.17 (1.52–11.11) | 0.004 |
| Ertapenem | 9 (31.0) | 10 (20.8) | 1.72 (0.60–5.00) | 0.230 |
| Imipenem or meropenem | 15 (51.7) | 14 (29.2) | 2.63 (1.00–7.14) | 0.042 |
| Overall mortality | 13 (44.8) | 26 (54.2) | 0.68 (0.27–1.73) | 0.288 |
OR, odds ratio; COPD, chronic obstructive pulmonary disease.
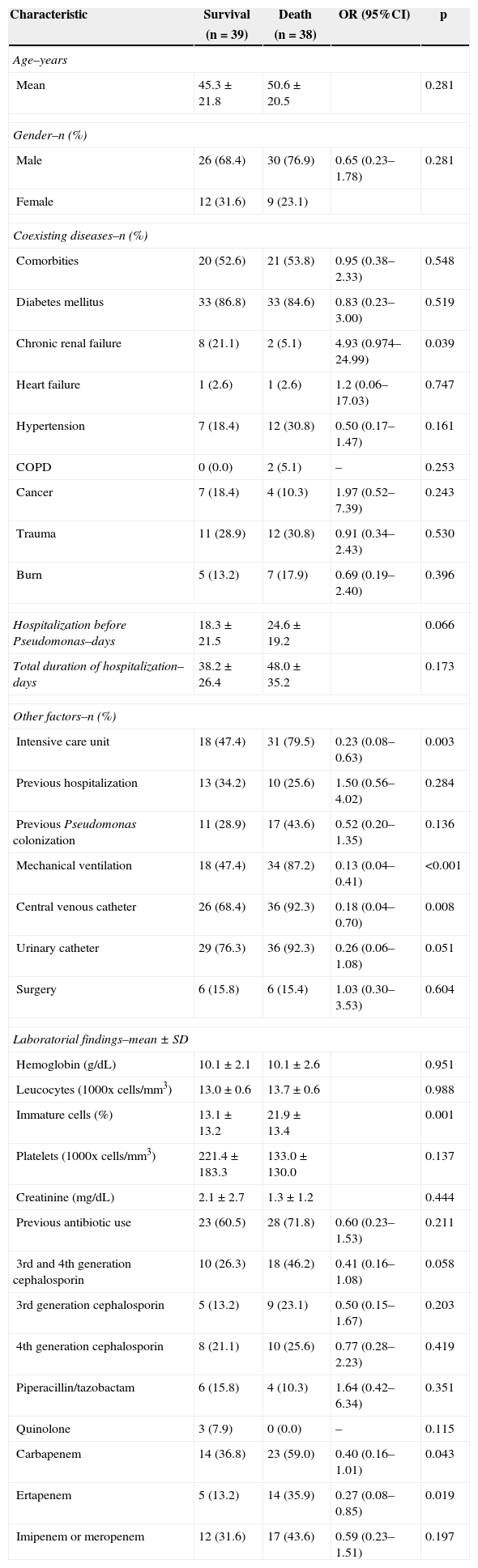
The 30-day mortality of patients with P. aeruginosa bloodstream infection was 44.8% for patients with CS-PA and 54.2% for patients with CR-PA, without statistical significance. The univariate analyses of factors associated with in-hospital mortality are listed in Table 2. Chronic renal failure, admission to the ICU, mechanical ventilation, and central venous catheter were risk factors for mortality. The percentage of immature white cells was higher in patients who died. The multivariate analysis did not single out any isolated risk factor for mortality of patients with P. aeruginosa.
Risk factors for mortality of patients with Pseudomonas aeruginosa bacteremia. Values are given as absolute number followed by percentage.
| Characteristic | Survival | Death | OR (95%CI) | p |
|---|---|---|---|---|
| (n=39) | (n=38) | |||
| Age–years | ||||
| Mean | 45.3±21.8 | 50.6±20.5 | 0.281 | |
| Gender–n (%) | ||||
| Male | 26 (68.4) | 30 (76.9) | 0.65 (0.23–1.78) | 0.281 |
| Female | 12 (31.6) | 9 (23.1) | ||
| Coexisting diseases–n (%) | ||||
| Comorbities | 20 (52.6) | 21 (53.8) | 0.95 (0.38–2.33) | 0.548 |
| Diabetes mellitus | 33 (86.8) | 33 (84.6) | 0.83 (0.23–3.00) | 0.519 |
| Chronic renal failure | 8 (21.1) | 2 (5.1) | 4.93 (0.974–24.99) | 0.039 |
| Heart failure | 1 (2.6) | 1 (2.6) | 1.2 (0.06–17.03) | 0.747 |
| Hypertension | 7 (18.4) | 12 (30.8) | 0.50 (0.17–1.47) | 0.161 |
| COPD | 0 (0.0) | 2 (5.1) | – | 0.253 |
| Cancer | 7 (18.4) | 4 (10.3) | 1.97 (0.52–7.39) | 0.243 |
| Trauma | 11 (28.9) | 12 (30.8) | 0.91 (0.34–2.43) | 0.530 |
| Burn | 5 (13.2) | 7 (17.9) | 0.69 (0.19–2.40) | 0.396 |
| Hospitalization before Pseudomonas–days | 18.3±21.5 | 24.6±19.2 | 0.066 | |
| Total duration of hospitalization–days | 38.2±26.4 | 48.0±35.2 | 0.173 | |
| Other factors–n (%) | ||||
| Intensive care unit | 18 (47.4) | 31 (79.5) | 0.23 (0.08–0.63) | 0.003 |
| Previous hospitalization | 13 (34.2) | 10 (25.6) | 1.50 (0.56–4.02) | 0.284 |
| Previous Pseudomonas colonization | 11 (28.9) | 17 (43.6) | 0.52 (0.20–1.35) | 0.136 |
| Mechanical ventilation | 18 (47.4) | 34 (87.2) | 0.13 (0.04–0.41) | <0.001 |
| Central venous catheter | 26 (68.4) | 36 (92.3) | 0.18 (0.04–0.70) | 0.008 |
| Urinary catheter | 29 (76.3) | 36 (92.3) | 0.26 (0.06–1.08) | 0.051 |
| Surgery | 6 (15.8) | 6 (15.4) | 1.03 (0.30–3.53) | 0.604 |
| Laboratorial findings–mean±SD | ||||
| Hemoglobin (g/dL) | 10.1±2.1 | 10.1±2.6 | 0.951 | |
| Leucocytes (1000x cells/mm3) | 13.0±0.6 | 13.7±0.6 | 0.988 | |
| Immature cells (%) | 13.1±13.2 | 21.9±13.4 | 0.001 | |
| Platelets (1000x cells/mm3) | 221.4±183.3 | 133.0±130.0 | 0.137 | |
| Creatinine (mg/dL) | 2.1±2.7 | 1.3±1.2 | 0.444 | |
| Previous antibiotic use | 23 (60.5) | 28 (71.8) | 0.60 (0.23–1.53) | 0.211 |
| 3rd and 4th generation cephalosporin | 10 (26.3) | 18 (46.2) | 0.41 (0.16–1.08) | 0.058 |
| 3rd generation cephalosporin | 5 (13.2) | 9 (23.1) | 0.50 (0.15–1.67) | 0.203 |
| 4th generation cephalosporin | 8 (21.1) | 10 (25.6) | 0.77 (0.28–2.23) | 0.419 |
| Piperacillin/tazobactam | 6 (15.8) | 4 (10.3) | 1.64 (0.42–6.34) | 0.351 |
| Quinolone | 3 (7.9) | 0 (0.0) | – | 0.115 |
| Carbapenem | 14 (36.8) | 23 (59.0) | 0.40 (0.16–1.01) | 0.043 |
| Ertapenem | 5 (13.2) | 14 (35.9) | 0.27 (0.08–0.85) | 0.019 |
| Imipenem or meropenem | 12 (31.6) | 17 (43.6) | 0.59 (0.23–1.51) | 0.197 |
OR, odds ratio; COPD, chronic obstructive pulmonary disease.
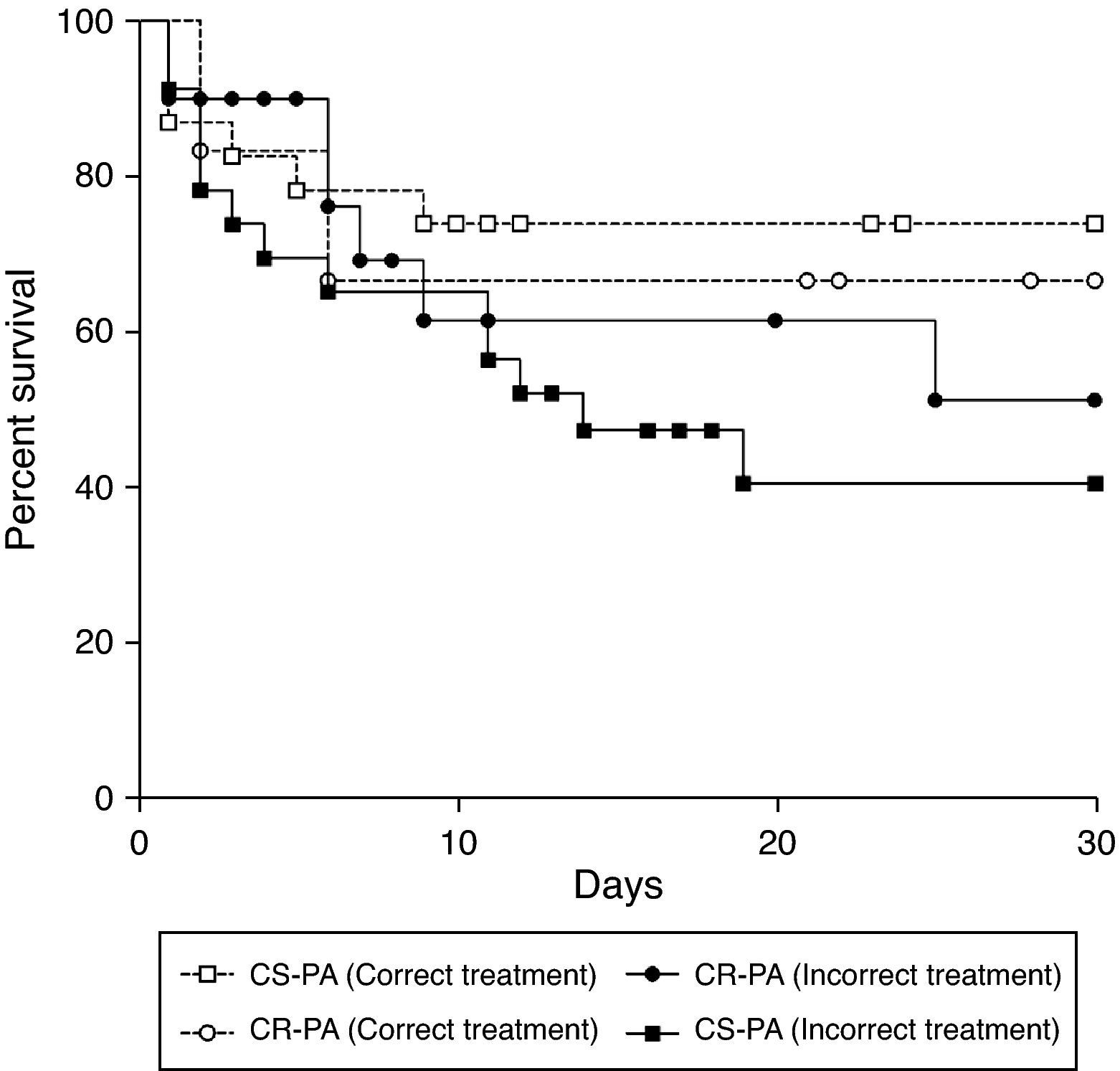
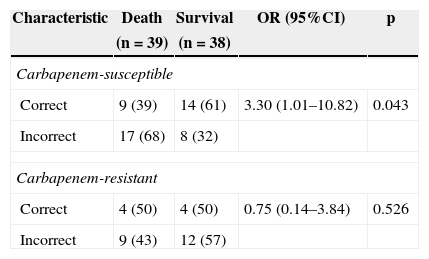
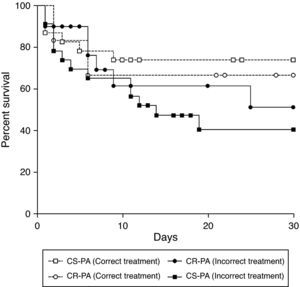
The treatment of bacteremia was incorrect in 46 of 77 patients (59.7%). Incorrect treatment increased mortality when compared to correct treatment of patients with bacteremia caused by CS-PA (39% vs. 68%; p=0.043), but not by CR-SA (43% vs. 50%; p=0.526) [OR: 3.30 (95% CI: 1.01–10.82; p=0.043] (Table 3). The mortality of patients with bacteremia caused by CR-PA was similar irrespective of correct or incorrect antimicrobial treatment. Fig. 1 shows the different pattern of survival curves among the four groups (correct and incorrect therapy for CS-PA and CR-PA). There was no difference among the groups, although there was a tendency of higher mortality in groups with incorrect therapy, including a higher discrepancy in the group of CS-PA, as detailed in Table 3.
Mortality of patients with Pseudomonas aeruginosa bacteremia comparing the correct therapy between carbapenem-resistant strains with those susceptible.
| Characteristic | Death | Survival | OR (95%CI) | p |
|---|---|---|---|---|
| (n=39) | (n=38) | |||
| Carbapenem-susceptible | ||||
| Correct | 9 (39) | 14 (61) | 3.30 (1.01–10.82) | 0.043 |
| Incorrect | 17 (68) | 8 (32) | ||
| Carbapenem-resistant | ||||
| Correct | 4 (50) | 4 (50) | 0.75 (0.14–3.84) | 0.526 |
| Incorrect | 9 (43) | 12 (57) | ||
Carbapenem has failed in the last years as a drug of choice in the treatment of nosocomial sepsis, considering the current resistance scenario. This group has published a recent study about the importance of including polymyxin as one of the drugs of choice in the empirical treatment of infections in hospitalized patients.15 Furthermore, the duration of hospitalization before bacteremia was not different between carbapenem-susceptible and carbapenen-resistant strains. However, the mean hospital length of stay before the first Pseudomonas bacteremia with CR-PA had a tendency to be greater. This finding was also reported in bacteremia caused by ESBL producing Enterobacteriaceae in the same hospital,16 suggesting that bacteremia by MDR bacteria occurs later. Unfortunately, the wide range of hospital length of stay before the first bacteremia does not allow for the determination of an ideal therapy considering only the day of hospitalization.
Patients with CR-PA were mainly in the ICU, under mechanical ventilation and with invasive procedures (e.g. central venous catheter). In a previous study invoving over 503 patients, independent risk factors for pan-resistant Pseudomonas bloodstream infection included previous transplantation, hospital-acquired infection, and prior ICU admission (OR 2.04; 95% CI: 1.15–3.63, p=0.015).17 The most interesting finding in the present study was the association with previous use of carbapenem, although this had already been described in the literature.18,19
The mortality of Pseudomonas bacteremia has been evaluated in large series of retrospective studies, including regional studies from Brazil. There was a tendency of higher mortality in the group of CR-PA in the literature, but not in all studies. Some of them do not attribute the mortality to the agent, but to the underline condition of the patient who developed a MDR Pseudomonas bacteremia.20 Another well-detailed characteristic of CR-PA is incorrect therapy. Zavascki et al. showed that metallo-beta-lactamase-producing P. aeruginosa increases the risk of incorrect therapy, increasing the mortality.21
The delay to begin the correct antibiotic therapy is associated with higher mortality in several studies among different species.22 This study demonstrated that this concept is not a constant, and is mainly associated with older patients and those with severe diseases as well as those infected with MDR bacteria.16,23 In the survival curve of the present study, there was a non-significant trend for higher mortality among those with incorrect therapy, probably due to small sample size in the subgroup analysis. All patients defined as receiving correct therapy started the drug in less than 24h after blood sample collection. The treatment options for CR-PA are scarce. Polymyxin B was the main drug used in this context, given that the samples in this hospital are pan-resistant. The dose of polymyxin B used in this institution is 25,000 IU/kg q12h, without dose adjust in the presence of renal failure.
This study confirmed these findings only for the CS-PA group. Probably, the CR-PA group did not show the decreased mortality due to severity of infection in the group with previous use of carbapenems, as demonstrated in the Table 1. It is suggested that there is a group of patients in which antibiotic therapy does not impact mortality.24,25
In conclusion, Pseudomonas bacteremia presents a high mortality, which can be reduced when correct therapy is employed, at least when carbapenem-susceptible strains are identified. Correct therapy does not necessarily change the evolution of CR-PA.
Conflict of interestAll authors declare to have no conflict of interest.