Among individuals coinfected with HCV and HIV, studies of mortality from non-hepatic causes have shown inconsistent results. The aim of this study was to investigate the contribution of HCV and HIV co-infection to mortality from hepatic and non-hepatic causes in Brazil. This retrospective cohort study included blood donors from Fundação Pró-Sangue de São Paulo (FPS) who were followed from 1994 to 2016 to compare mortality and its causes between HIV-HCV coinfected individuals versus those seronegative for all tested infections. Records from the FPS database and the Mortality Information System were linked through a probabilistic record Relationship (RL). The Hazard Ratio (HR) was estimated using Cox multiple regression models. HCV-HIV coinfected individuals compared to seronegative individuals had a higher risk of death from all causes (HR = 14.54), non-liver neoplasms (HR = 2.55), infections (HR = 10.37) and liver disease (HR = 7.0). In addition, HCV mono-infected individuals compared to seronegative individuals had a higher risk of death from all causes (HR = 2.23), liver cancer (HR = 32.21), liver disease (HR = 14.92), infection (HR = 3.22), and trauma (HR = 1.68). Individuals coinfected with HCV and HIV have increased overall mortality and death due to infections, liver diseases and non-liver neoplasms as compared to those uninfected with HCV and HIV.
Hepatitis C Virus (HCV) infection is a global public health problem, since 1 % of the world's population is infected with this virus, and about 80 % of cases have chronic disease.1 Although the World Health Organization established a global strategy in 2016 to eliminate hepatitis B and C viral infections, the diagnosis of asymptomatic chronic infection remains a challenge.1
Coinfection with HCV is associated with increased morbidity and mortality in HIV+ individuals.2-5 This fact becomes relevant when we take into consideration that there are more than 2.3 million individuals coinfected with HIV and HCV worldwide, representing about 6 % of the Population Living with HIV/AIDS (PLWHA).2,3 Moreover, mortality rate in coinfected individuals (HIV/HCV) is 12 times higher compared to the general population.2,4,5 This may be linked to the enhanced rate of HCV replication which speeds up disease progression and the occurrence of cirrhosis, in addition to increasing mortality.6
Considering that Brazil has approximately 700,000 people with chronic HCV infection and, since 2007, 381,793 new cases of people living with HIV, it is important to assess the impact of the association of these diseases on the risk and causes of death.7,8 The amount of HIV-HCV co-infected individuals is difficult to estimate due to lack of good quality data. Nevertheless, it is important to assess mortality rates among coinfected individuals. To date, detailed analyses of cause-specific mortality among HIV-HCV-infected persons remain limited. The present study follows a cohort of blood donos with known serological status who underwent an interview before donation and were in good health. We evaluated the impact of HCV and HIV coinfection on risk and causes of death, based on a large cohort of blood donors. We analyzed risk factors for all-cause and cause-specific mortality.
Material and methodsData sourcesIndividual data of the FPS regarding blood donors between 1994 and 2013 were linked to mortality data up to 2016 of the SIM databases. The FPS database contained information regarding identification ‒ individual name, mother's name, date of birth, sex, and address ‒ and information regarding HCV and HIV infection status. Mortality information in the SIM database is based on the international form of the death certificate and Cause Of Death (COD), and is assigned by the attending physicians, pathologists of the Death Investigating Services (SVOs), or coroners from the Forensic Institutes (IMLs). The International Classification of Diseases, 10th Edition (ICD-10) codes are used to classify COD which is reviewed by certified coders.9–12 The identification variables of the SIM database – individual name, mother's name, date of birth, address, sex, and age – were used to link with the FPS database, while the variables Date of Death and CID-10 Codes for the underlying and associated causes of death were used for the analysis.
Study populationHCV monoinfected and HCV-HIV coinfected blood donors between 1994 and 2013, plus a random sample of HCV and HIV-negative donors were included in the analysis. During the blood donation period (1994‒20132) FPS screened all donors for HCV infection by enzyme-linked immunosorbent assay (ELISA) and RIBA HCV 3.0 strip immunoblot assay.14 HCV-positive donors were defined as those who tested positive for EIA with RIBA HCV 3.0 or Western blot followed by a positive EIA.13,15-17 Nucleic Acid Amplification Tests (NAAT) for HCV diagnosis were not required in Brazil until November 2013.
The FPS and SIM databases lack information regarding lifestyle habits, socioeconomic conditions, and other health risks behaviors, like tobacco or alcohol use or the presence of other co-morbidities. Also, in the databases accessed there was no information on clinical or laboratory characteristics, such as liver function, HIV CD4+, and CD8+ T-cell, HIV, or HCV viral load data. Also, there was no information on how many of these individuals’ received treatment for HIV or HCV infection, during follow-up.
Record linkage proceduresDue to the absence of a national identity number, a two-step Record Linkage (RL) procedure was used. First, an in-house linkage algorithm was developed by staff members of the Brazilian Ministry of Health in which the probability that the two records would belong to the same patient was based on the person's name and date of birth. A Bloom filter, a space-efficient probabilistic data structure, was constructed for each record following the methods developed by Schnell et al.18 Because high sensitivity was achieved in this step at the expense of low specificity, a second step was necessary to increase the specificity of the matches found in the first step. The second step was performed by the study researchers using the Reclink III open-source software.19 Patient's names, mother'snamese, and dates of birth were chosen as the matching variables. Three blocking step strategies were used based on a combination of the phonetic codes of the variable's first name, last name, and sex. The first blocking step used sex and the phonetic codes of the first name and last name. The second blocking step used only sex and the phonetic code of the first name, and the third blocking step used only sex and the phonetic code of the last name. The RL procedure used has been validated in a previous publication20 and achieved a sensitivity of 94 % (95 % CI 90 %–97 %) and a specificity of 100 % (95 % CI 98 %‒100 %).
Unfortunately, the SIM database contained mortality information only beginning in 2000, so the researchers could not perform RL between FPS and SIM for earlier periods. For the cohort that donated blood in 1994, we were unable to consider the first six years of follow-up. For the 1995 cohort, we were unable to consider the first five years of follow-up. In other words, regardless of the patient's HCV serology status, we do not know whether those who donated blood in 1994, for example, died in the period between 1994 and 1999. However, based on findings of seropositive and seronegative individuals who donated blood in 1999 and from whom we lacked data only on deaths that may have occurred during the year of their blood donation, we found that deaths were extremely rare in the first year after donation. Because of this finding and to work around this limitation to the data, we made the analytical assumption that no one in our cohort died between 1994 and 1999.
Statistical analysisTotal and proportional amounts were used to describe the database while Pearson's chi-square test was used to verify group differences for categorical data. Univariate Cox regression models were used to estimate Hazard Ratio between the following groups: seronegative vs HCV+HIV+; HCV+ vs seronegative. Moreover, Multivariate Cox regression models were used to estimate Hazard Ratios between the same groups described above, considering covariates Age Group and Sex. The proportional hazards assumption was verified by visualization of parallelism in a log-log graph and by scaled Schoenfield residuals. The level of confidence is 95 %, while statistical significance is achieved when p-values were < 0.05.
Ethical approvalThe study was approved by the Research Ethics Committee of the Clinical Hospital of the University of Sao Paulo School of Medicine, or HC-FMUSP (CAAE Registry n° 62572616.5.0000.0065). The committee deemed it unnecessary to obtain informed consent from each individual for the review of their medical records due to the difficulty of obtaining such consent and the retrospective nature of the two databases. All individual identifiers were removed from the database after the probabilistic RL was performed.
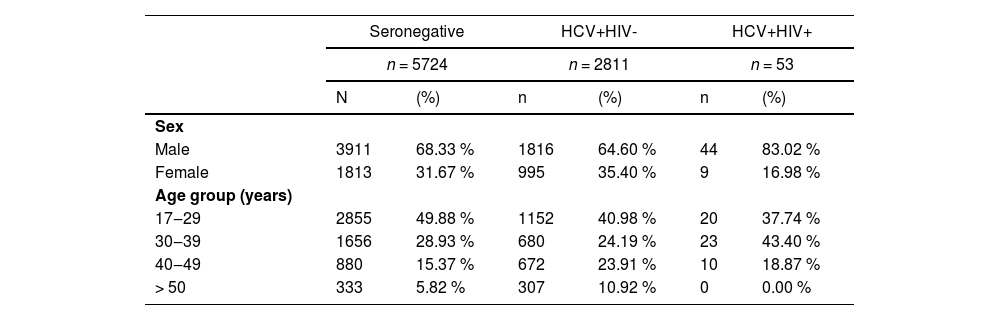
ResultsThe study population is composed by 8588 individuals, of which 5724 (66.65 %) were HCV- HIV-, 2811 (32.73 %) were HCV+ HIV- and 53 (0.62 %) were HCV+ HIV+. Each group was composed mainly of males between 17 and 39 years old. The HCV+HIV+ group had the largest difference in sex composition, with 83.02 % males and 16.98 % females. In addition this group was composed mainly of individuals between 30 and 39 years old (43.40 %). In contrast, the seronegative and HCV+HIV- groups were both composed mainly of individuals between 17 and 29 years old (49.88 % and 40.98 %, respectively). Details are presented in Table 1.
Characteristics of blood donors according to HCV and HIV status.
Chi-Square test for the variables Sex and Age Group were statistically significant (p < 0.05) in all comparisons (Seronegative vs. HCV+HIV-, Seronegative vs. HCV+HIV+, HCV+HIV- vs. HCV+HIV+).
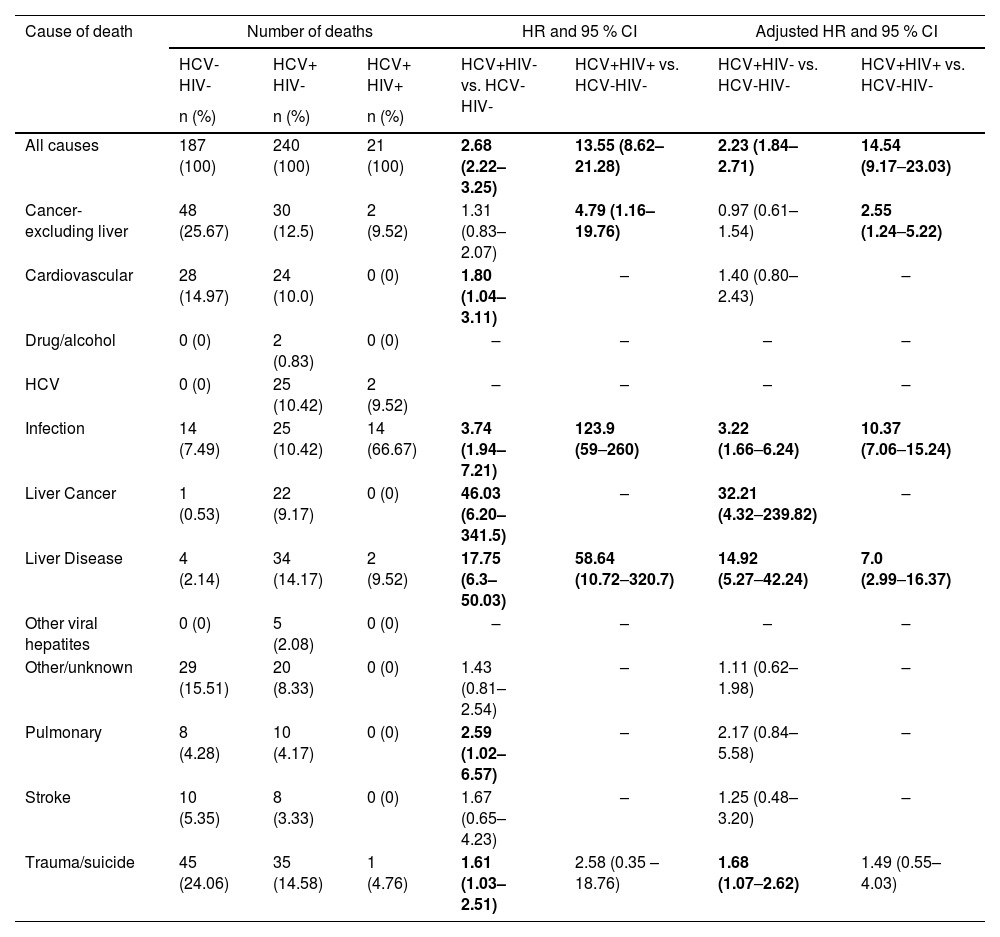
In the study period, 3.3 % of the HCV-HIV- individuals, 8.5 % of the HCV+HIV- and 39.6 % in the HCV+HIV+ died. In the HCV-HIV- group, non-liver cancer (25.7 %) and Trauma/Suicide (24.1 %) accounted for almost 50 % of all deaths, while in the HCV+HIV- group liver-related conditions (Liver Disease, HCV, and Liver Cancer) combined were responsible for 33.75 % of all deaths. Regarding the HCV+HIV+ group, infection was responsible for the majority of deaths (66.7 %).
Considering all causes of death, the univariate survival analysis revealed an increased risk of death for the HCV+HIV+ (HR = 13.55, 95 % CI 8.62–21.28) group when compared to HCV-HIV- subjects. Correcting for Sex and Age Group in the multivariate analysis, the group HCV+HIV+ maintained an increased risk of death (HR = 14.54, 95 % CI 9.17–23.03) in comparison to those who were HCV-HIV-.
The univariate survival analysis by Cause of Death revealed that the HCV+HIV+ group had an increased risk of death for all causes in comparison to HCV-HIV- subjects, by non-liver cancer (HR = 4.79, 95 % CI 1.16–19.76), Infection (HR = 123.9, 95 % CI 59‒260) and Liver Disease (HR = 58.64, 95 % CI 10.72–320.7). In the multivariate analysis the HCV+HIV+ group had an increased risk of death in comparison to the HCV-HIV- group, for non-liver cancer (HR = 2.55, 95 % CI 1.24‒5.22), Infection (HR = 10.37, 95 % CI 7.06‒15.24) and Liver Disease (HR = 7.0, 95 % CI 2.99‒16.37). Details are presented in Table 2.
Absolute and relative number of deaths and survival analysis by Infection Status and Causes of Death.
Univariate and Multivariate Cox regression models allowed the calculation of Hazard Ratio (HR) with 95 % CI. Sex and Age Groups were considered covariates in the Multivariate analysis. The bold values indicate statistical significance (p < 0.05).
In addition, by multivariate analysis members of the HCV+HIV- group had an increased risk of death from all causes (HR = 2.23, 95 % CI 1.84–2.71), Infection (HR = 3.22, 95 % CI 1.66‒6.24), Liver Cancer (HR = 32.21, 95 % CI 4.32‒239.82) 32.21, Liver Disease (HR = 14.92, 95 % CI 5.27‒42.24), and Trauma (HR = 1.68, 95 % CI 1.07‒2.62) in comparison to the HCV-HIV- group (Table 2).
DiscussionIn the present study, in which a cohort of blood donors was followed for 22 years (1994 to 2016), it was observed that HIV-HCV coinfection was associated with increased overall mortality and death due all causes, both liver-related and non-liver related, as compared to individuals infected only with HCV or HCV- HIV- subjects.
Multiple studies have sought to quantify the burden of mortality from HCV. However, to our knowledge, this is the first data linkage study which aimed to describe mortality and liver and non-liver causes of death among HIV-HCV coinfected individuals as compared to the non-HIV-HCV population.
In the study period, 39.6 % of the HCV+HIV+ and 3.3 % of the HCV-HIV- individuals died. Among the HCV+HIV+ cohort, infection was responsible for the majority of deaths (66.7 %). Correcting for sex and age in the multivariate analysis, in addition to infection, the risk of death was significantly higher for liver disease and non-liver cancer when the HCV+HIV+ group was compared to the seronegative group. Our data confirm what has been described previously, that HCV and HIV co-infection enhances the risk of death.2-5,21-23
Our findings may be explained by the presence of comorbidities and specific habits, such as alcohol consumption and injection drug use frequently described among people living with HIV-AIDS and less frequently described in the general population. This may account for the increase of non-liver deaths in our cohort.
As already mentioned, when the underlying causes of death were categorized, the presence of infection greatly increased the risk of death in co-infected individuals as compared to seronegative individuals. The association of HCV or HIV infection when considered individually, with sepsis, community-acquired pneumonia and tuberculosis is well known,24-27 as is the association of HIV infection with immunosuppression.28
Our findings related to the presence of infection as a cause of death in this population are in accordance with these data. The risk of death from non-liver neoplasms, from liver disease and from HCV- associated conditions, previously reported,28-38 were also frequent in our HCV+ HIV+ cohort. The occurrence of non-hepatic neoplasms in these subjects might have been influenced by HIV coinfection due to chronic inflammation and immune system dysregulation caused by the HIV infection.38
Direct-acting antivirals as treatment for HCV and HIV slow the progression of liver disease and reduce the risk of liver cancer and death among infected individuals.38,39 However, there may still be a higher risk of death,38,40 as was also observed in our cohort.
After a blood donor is identified as HIV or HCV infected, treatment and follow-up are offered freely through the Brazilian national health care system (Sistema Único de Saúde). We do not have information on how many of these individuals were properly treated after being diagnosed with these infections. Nevertheless, it is reasonable to suppose that a substantial part of the study population may have received treatment. This possibility raises two questions: first, the mortality rates observed here may be a reflection of treatment and, therefore, the impact of HCV and HIV chronic infection in mortality may be even greater. In addition, as discussed previously,38,40 HCV treatment does not eliminate the risk of death due to HCV-related causes.
Among HCV monoinfected individuals, liver disease, hepatitis C and liver cancer combined accounted for 33.75 % of all deaths. HCV monoinfected individuals compared to seronegative individuals had a higher risk of death from all causes (HR = 2.23), liver cancer (HR = 32.21), liver disease (HR = 14.92), infection (HR = 3.22), and trauma (HR = 1.68). Since HCV is predominantly asymptomatic and is not routinely tested for in the general population, our data reinforces the need of pursuing diagnosis and treatment of HCV-infected individuals in Brazil.
Since 2018, Brazil extended treatment to everyone with a HCV diagnosis and launched a range of other initiatives focused on diagnosis and prevention. This put Brazil at the forefront in addressing HCV infection. Monitoring this strategy is essential to achieving success with the Brazilian HCV macroelimination plan. Also, it would be plausible to suppose that emphasizing a plan focused on HCV microelimination among high-risk populations like HIV coinfected individuals would make HCV macroelimination in Brazil faster and more feasible.
Limitations of the present study need to be acknowledged. In the databases accessed there was no information on lifestyle habits, the presence of other comorbidities and socioeconomic conditions which could have influenced mortality in our cohort.41 Also another limitation is the fact that we did not have access to data on monoinfected HIV-patients mortality, therefore we were not able to compare mortality among HCV/HIV vs. monoinfected HIV-patients. Also it is possible that the number of AIDS or liver-related deaths might be overestimated in coinfected individuals as a known AIDS or hepatitis diagnosis could influence how a death is reported in the death certificate. Moreover, the collection of data ending in 2013 is another limitation, inasmuch as from that year on the impact of HCV Direct-Acting Antivirals (DAAs) on morbidity and mortality from HCV was notable in HCV monoinfected and HIV-HCV coinfected populations.
Despite these limitations, the present study utilized a large cohort of blood donors and HCV infected individuals and, as such, provides novel estimates of mortality among HIV and hepatitis-coinfected individuals in Brazil. It should also be stressed that our study was performed on potential blood donors, who, in addition to reflecting the characteristics of this population, are usually in good health at the time of donation.
Data linkage between laboratory diagnosis and death data is a highly relevent tool for healthcare planning, monitoring and evaluation of services. Changes in mortality trends in this population could help evaluate the impact of HIV and HCV treatments and other interventions to achieve HCV elimination as proposed by the WHO global strategy.
ConclusionIn a large Brazilian cohort of blood donors followed for 22 years, seropositivity for both HCV and HIV was associated with an increase in the overall rate of death and, especially, on deaths due to liver diseases, non-liver neoplasms and infections.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Dr. Steven S. Witkin reviewed the final manuscript for English grammer and clarity of expression.