The aim of this study was to compare both the efficacy and safety profile of the WHO-recommended, dual therapy (doxycycline–rifampin) to a quinolone-based, triple therapy (doxycycline–rifampin–levofloxacin) for treating acute/subacute brucellosis.
Patients and methodsWe studied 107 consecutive, naïve patients with acute/subacute brucellosis admitted to Assiut University Hospital. Patients were randomly allocated to receive the dual therapy of doxycycline–rifampin (group-A) or to receive the triple therapy of doxycycline–rifampin–levofloxacin (group-B). Acute/subacute brucellosis was diagnosed based on the presence of: (1) contact with animals or fresh animal products, (2) suggestive clinical manifestations of less than one-year duration, and (3) positive antibody titer (1:160) by standard tube agglutination test.
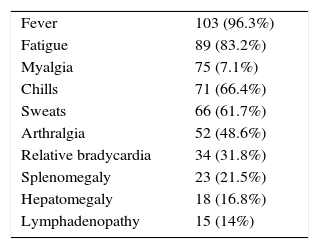
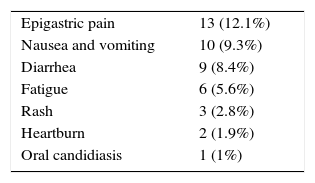
ResultsThere was no significant difference between the two groups regarding their demographic data. Fever was the most frequent manifestation (96.3%). Epigastric pain was the most frequent adverse effect of treatment (12.1%). Group-A patients had a significantly higher relapse rate compared to group-B patients (22.6% versus 9.3%, p-value=0.01). The rate of treatment adverse effects was higher among group-B patients, although not reaching statistical significance (20.4% versus 11.3%, p-value=0.059).
ConclusionsAdding levofloxacin to the dual therapy for acute/subacute brucellosis (doxycycline–rifampin) may increase its efficacy in terms of lowering the relapse rate of the disease. Further, larger scale studies are needed before considering modifying the standard, dual therapy for brucellosis.
More than 500,000 cases of brucellosis are reported yearly to the World Health Organization (WHO) from 100 countries. Seroprevalence of brucellosis in Assiut Governorate (Egypt) was estimated to be 1.29% among the general population.1 Bacteria of the genus Brucella cause disease with protean manifestations. Infection is transmitted to humans from animals as a consequence of occupational exposure or ingestion of contaminated milk products. Brucella abortus infection occurs worldwide with a reservoir in cattle. It is usually associated with mild to moderate sporadic disease; suppurative or disabling complications are rare. Brucella melitensis, with a reservoir in sheep, goats, and camels may cause severe, acute disease and disabling complications. It accounts for the majority of cases, distributed primarily in the Mediterranean region, Latin America, the Arabian Gulf, and the Indian subcontinent. Clinically, human brucellosis can be conveniently but arbitrarily divided into subclinical illness, acute or subacute disease, localized disease and complications, relapsing infection, and chronic disease. The WHO recommends doxycycline (200mg/day) plus rifampin (600–900mg/day) orally for six weeks for treating brucellosis. Up to 10% of patients with brucellosis experience relapses after antimicrobial therapy. Relapses usually occur three to six months after completion of therapy but may be seen up to two years after treatment.2
Combination drug therapy of brucellosis leads to shortening duration of symptoms, and decreases morbidity while, single drug therapy is associated with more relapse episodes and a higher rate of drug resistance.3 Therapeutic failure and relapse develop in 8% and 16%, respectively, of patients with acute brucellosis receiving doxycycline plus rifampin for 45 days compared to 2% and 5.2% among patients receiving doxycycline plus streptomycin for the same duration.4 Although regimens with aminoglycosides have higher therapeutic success rates, long-term use of such agents is associated with significant nephrotoxicity.
The use of quinolones for treating brucellosis is controversial. Reported by several studies, monotherapy with ciprofloxacin results in an unacceptably high probability of relapse.5–7 In four out of six randomized, controlled trials, ofloxacin was the quinolone used in combination with rifampin. In three of these studies, the results were similar between the quinolone and the non-quinolone arms regarding initial treatment success and probability of relapse.8–10 In one study of with spondylitis exclusively, the quinolone arm was found to be inferior to other treatment regimens in terms of initial treatment success and relapse rate.11 In two randomized, controlled trials, ciprofloxacin was the quinolone used, combined with doxycycline12 or rifampin13; the results were similar between the quinolone and the non-quinolone groups.
TheoryThere are no studies in Egypt investigating the use of triple antimicrobial therapy for brucellosis. The aim of this study was to compare both the efficacy and safety profile of the WHO-recommended, dual antimicrobial therapy (doxycycline–rifampin) to a quinolone-based, triple therapy (doxycycline–rifampin–levofloxacin) for treating acute/subacute brucellosis among Egyptian patients.
Patients and methodsThe study included 120 consecutive, naïve patients with acute/subacute brucellosis who had not received any antimicrobial therapy since the start of illness. The patients were admitted to the departments of Tropical Medicine (Fever Unit) and Internal Medicine during the period of May 2011 to November 2014. Acute/subacute brucellosis was diagnosed based on the presence of: (1) contact with animals or fresh animal products, (2) suggestive clinical manifestations of less than one-year duration (fever, chills, sweats, fatigue, arthralgia, myalgia, relative bradycardia, splenomegaly, lymphadenopathy, and hepatomegaly), and (3) positive antibody titer (1:160) by standard tube agglutination test (against Brucella abortus, Brucella melitensis, and Brucella suis). Pregnant and pediatric patients were excluded from the study. We defined therapeutic failure as persistence of the clinical manifestations of brucellosis at end of treatment (after six weeks of treatment). Relapse was defined as recurrence of the clinical manifestations with a single positive antibody titer within six months after ending therapy.2
The study patients were randomly allocated to two groups; 60 patients for each group. Group-A patients received the WHO-recommended, dual antimicrobial therapy for brucellosis (doxycycline 200mg/day and rifampin 900mg/day, for six weeks). Group-B patients received quinolone-based, triple therapy (doxycycline 200mg/day, rifampin 900mg/day, and levofloxacin 500mg/day, for six weeks). Each drug used was produced by same pharmaceutical company and all drugs were administered orally, once daily. Doxycycline was given after breakfast; rifampin was given before breakfast; and levofloxacin was given 1h before lunch. During inpatient attendance, the patients were assessed clinically on a daily basis; after discharge, they were assessed weekly on outpatient clinic visits. Complete blood count, liver chemistry panel, kidney chemistry panel, abdominal ultrasonography, and chest radiography were performed before the start of treatment. Laboratory investigations were repeated weekly to monitor for side effects. Directly observed therapy was applied during the period of inpatient attendance for all patients. The patients were discharged after normalization of body temperature for at least three days. After discharge, compliance with therapy was confirmed on weekly basis during outpatient clinic visits. After the end of therapy, the patients were assessed clinically on a monthly basis for six months to look for evidence of relapse.
Ethical considerationsThe study was approved by the “Assiut Faculty of Medicine Clinical Research Ethical committee”, and was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Before enrollment in the study, all participants signed an informed consent. Before signing, they were not able to discuss in details with the investigator the informed consent issues and the study aim. The participants were clearly informed that refusing to participate in the study would not affect having full benefit of the available medical service and treatment. Data were collected by personal interview with the participants taking in consideration data confidentiality.
Statistical analysisThe data were entered into and then were analyzed using the SPSS (version 22). Results were expressed as mean±standard deviation or frequency (percentage) as appropriate. The Chi-square test or Fisher's exact-test was used to analyze the difference among categorical variables. Student's t-test or Mann–Whitney U test were used to analyze numeric variables as appropriate. A p-value of less than 0.05 was considered significant.
ResultsOut of the study population (120 patients), five were excluded due to lack of compliance with antimicrobial therapy for brucellosis (two patients of group-A and three of group-B). Eight more patients were excluded due to inability to attend during the required follow up period (six months after the end of treatment) (five of group-A and three of group-B). After exclusion of such cases, group-A consisted of 53 patients while group-B had 54. Demographic data of the study population (107 patients) according to the type therapy are shown in Table 1. The mean age was 32.7±15 and 36.2±17.4 years, respectively for group-A and group-B. There were 32 (60.4%) males in group-A and 30 (55.6%) in group-B. There was no significant difference between the two groups regarding their demographic data.
The clinical manifestations of acute/subacute brucellosis in the study population are shown in Table 2. The most common clinical manifestation was fever (96.3%).
Outcomes of antimicrobial therapy for brucellosis in the study population included therapeutic failure in 13 patients (12.1%), relapse in 17 (15.9%), and adverse effects of treatment in 17 (15.9%).
The adverse effects of antimicrobial therapy for brucellosis in the study population are shown in Table 3. The most commonly reported adverse effect was epigastric pain (12.1%). Some patients had more than one adverse effect either concomitantly or sequentially. No serious adverse effects were reported among patients of either group.
The outcomes of antimicrobial therapy according to the type of therapy used are shown in Table 4. Although the rate of therapeutic failure was slightly higher in group-A (13.2%) compared to group (B) (11.1%), this difference was not statistically significant (p-value 0.394). However, the relapse rate was significantly higher in group-A (22.6%) compared to group-B (9.3%) (p-value 0.010). Regarding the safety profile of the used regimens, the rate of adverse effects was higher in group-B (20.4%) compared to group-A (11.3%), although this difference did not reach statistical significance (p-value 0.059).
Outcome of antimicrobial therapy for brucellosis according to the type of therapy (n=107).
| Outcome | Group (A) (n=53) | Group (B) (n=54) | p-value |
|---|---|---|---|
| Therapeutic failure | 7 (13.2%) | 6 (11.1%) | 0.394 |
| Relapse | 12 (22.6%) | 5 (9.3%) | 0.010a |
| Adverse effects | 6 (11.3%) | 11 (20.4%) | 0.059 |
n, number.
Human brucellosis has a major medical impact world-wide, and its eradication deemed to be difficult.14 With a seroprevalence of 1.29% among the general population in Assiut Governorate (Egypt),1 brucellosis could be considered an important public health issue in Egypt. The appropriate antimicrobial therapy of brucellosis will reduce morbidity, prevent complications, and diminish relapses.15 Up to 10% of patients with brucellosis relapse after antimicrobial therapy. The intracellular location of Brucella organisms predisposes to recurrence because the organisms are relatively protected from host defense mechanisms, and antimicrobial agents may be unable to penetrate efficiently enough to kill all the bacteria.2 In vitro studies have demonstrated resistance of Brucella to rifampin.16,17 In Egypt, Abdel-Maksoud et al. reported resistance to rifampin and ceftriaxone among 355 Brucella isolates analyzed that were susceptible to tetracycline, doxycycline, trimethoprim-sulfamethoxazole, streptomycin, and ciprofloxacin.18
The main goal of this randomized, comparative study was to compare the efficacy and safety of the WHO-recommended, dual antimicrobial therapy for acute/subacute brucellosis (doxycycline–rifampin) to a quinolone-based, triple antimicrobial therapy (doxycycline–rifampin–levofloxacin). In the present study, the use of triple antimicrobial therapy for brucellosis has yielded both higher rate of persistent cure and lower relapse rate within six months after stopping therapy compared to the dual antimicrobial therapy (9.3% versus 22.6%) among the study population, in spite of nearly equal rates of clinical response and therapeutic failure after ending six weeks of therapy (11.1% versus 13.2%). However, the relapse rate among the study patients who received the dual antimicrobial therapy was much more higher than that reported by Akova et al. (22.6% versus 3.3%),8 and also higher than that reported by Karabay et al. (14.3% versus 22.6%),9 and Solera et al. in patients with acute brucellosis who received the same treatment regimens (22.6% versus 16%)4 which raises concerns about the increasing development of microbial resistance to the dual antimicrobial therapy for brucellosis in Egypt. The lower efficacy of the dual antimicrobial therapy could not be attributed to any difference between the two groups of the present study.
In this study both regimens of antimicrobial therapy for brucellosis (dual and triple) were considered safe and well-tolerated. No serious adverse effects of treatment were found. The rates of adverse effects in both group-A (11.3%) and group-B (20.4%) patients were lower than that reported among patients who received rifampin and doxycycline (43.3%) although it was higher than that of patients who received rifampin and ofloxacin (6.5%) reported by Akova et al.8 Only five (4.2%) out of 120 patients could not tolerate antimicrobial therapy for brucellosis and thus were excluded from the study, with nearly no difference between the two groups (two out of 60 patients receiving the dual therapy and three out of 60 patients receiving the triple therapy).
As far as we know, no previous study had evaluated the triple antimicrobial therapy (doxycycline plus rifampin plus levofloxacin) for treating brucellosis. One study has investigated a different triple therapy for acute brucellosis. Ranjbar et al. found that doxycycline–rifampin–amikacin has resulted in a lower relapse rate (5.7%) compared to the dual antimicrobial therapy (doxycycline–rifampin) (9.3%) with higher rate of side effects in patients who received triple (5.5%) compared to those who received dual antimicrobial therapy (3.6%), although the rates difference did not reach statistical significance.19 Previous studies of dual antimicrobial therapy for treating brucellosis revealed varying relapse rates when using rifampin combined with a quinolone. For use of ofloxacin combined with rifampin, relapse rates ranged between 3.2%8 and 14.3%,9 and 15% when ciprofloxacin was combined with rifampin.13 Such results imply that using a quinolone combined with rifampin for treating brucellosis usually yields higher relapse rates compared to using a quinolone combined with rifampin and doxycycline such as the triple antimicrobial therapy used for patients of group-B in our study.
The present study has some limitations. The study sample is relatively small which was determined by availability of cases diagnosed during the study period. We did not use culture for confirming the serologic diagnosis; microbiological hazard of culturing Brucella organism made this choice a remote one. Also, in spite of the instructions provided for the study population to avoid contact with animals or fresh animal products, compliance could not be guaranteed making re-infection a potential cause for disease recurrence beside relapse. However, exposure rates were similar among both groups of the study. Also, in this study relapse was not confirmed by a rising antibody titer; rather, it was based on recurrence of the clinical manifestations with a single positive antibody titer. Although we assumed no antimicrobial therapy before admission, this was based on both patients and/or his/her relatives information in addition to revising the patients’ prescriptions, this is not a 100% guarantee of not having used antimicrobials before admission.
ConclusionsIn conclusion, we found a higher than average relapse rate of brucellosis in Egypt. Adding levofloxacin to the WHO-recommended, dual antimicrobial therapy for acute/subacute brucellosis (doxycycline–rifampin) may increase its efficacy in the terms of lowering relapse rates within six months after stopping therapy. However, it had no effect on the rate of therapeutic failure after six weeks of therapy. The rate of adverse effects for triple antimicrobial therapy for brucellosis was slightly higher, but it did not affect patient compliance or jeopardize safety. Further, larger scale studies are needed before considering modifying the standard, dual antimicrobial therapy for brucellosis in Egypt.
Conflicts of interestThe authors declare no conflicts of interest.