There is an increasing number of older patients with human immunodeficiency virus infection due to the success of antiretroviral therapy, the improved prognosis and life expectancy of patients, and the higher number of new infections among older individuals. The main objective of the present study was to compare the characteristics of older human immunodeficiency virus patients with those of younger patients.
Materials and methodsWe conducted a cross-sectional study with human immunodeficiency virus-infected patients who were treated at the Specialized Care Service (Serviço de Assistência Especializada) for human immunodeficiency virus/AIDS in the city of Pelotas, South Brazil. Sociodemographic information as well as data on human immunodeficiency virus infection and treatment were collected. All participants underwent psychiatric and neurocognitive assessments, and their adherence to antiretroviral therapy was evaluated.
ResultsA total of 392 patients participated in the study, with 114 patients aged 50 years and older. The characteristics showing significant differences between older and younger human immunodeficiency virus-infected patients included race/ethnicity, comorbidities, duration and adherence to antiretroviral therapy, currently undetectable viral load, and cognitive impairment. Compared to younger patients, older patients were at higher risk of exhibiting cognitive impairment [OR 2.28 (95% CI: 1.35–3.82, p=0.002)] and of having increased adherence to antiretroviral therapy [OR 3.11 (95% CI: 1.67–5.79, p<0.001)].
ConclusionsThe prevalence of neurocognitive impairment remained high in human immunodeficiency virus-infected patients despite antiretroviral therapy. In the present study, the prevalence of this type of impairment was significantly higher in patients aged ≥50 years, most likely due to aging, human immunodeficiency virus infection, and a possible synergistic effect between these factors. Despite this higher prevalence, older patients exhibited higher rates of adherence to antiretroviral therapy and of undetectable human immunodeficiency virus viral load.
With the advent of antiretroviral therapy (ART), the prognosis of human immunodeficiency virus (HIV)-infected individuals has dramatically improved. The evolution of this therapy into less complex regimens that are safer, easier to administer, and have fewer side effects allows for viral replication to be controlled indefinitely in most patients.1,2 Despite the success of ART, which led to the reduction of HIV-related opportunistic diseases, HIV-infected individuals exhibit an increase in non-AIDS-defining illnesses that are typically related to aging, such as cardiovascular diseases, dyslipidemia, diabetes mellitus, cancer, liver diseases, renal diseases, bone disorders, and neurocognitive impairment (NCI).3,4 Aging is accelerated in HIV-infected patients compared to non-HIV-infected patients, with subjects aged 50 years and above being considered elderly.4–7 Several factors have been associated with the above-mentioned differences in aging, including chronic HIV infection, ART side effects, and accelerated aging of the immune system.8–10
NCI in HIV-infected patients has remained highly prevalent even after the advent of ART. In addition to changes associated with HIV (HIV-associated neurocognitive disorder – HAND),11,12 other factors traditionally related to cognitive impairment are present in HIV-infected patients and have a significant association with cognitive impairment in this population. The contribution of aging to the persistence of NCI has driven growing interest in the study of patients using ART. Several reports have shown a higher prevalence of cognitive impairment among older HIV-infected patients compared to younger patients using ART.13–16
There has been an increase in the population of older HIV-infected adults due to increased number of new HIV infections at more advanced ages, the chronic nature of the infection, and increases in life expectancy.17,18 Further studies are necessary to better understand this population. The objectives of this study were to (i) compare the characteristics of younger HIV-infected patients with those of the older patients; (ii) assess the prevalence of cognitive impairment in HIV-infected patients after the advent of ART; (iii) determine whether an association exists between cognitive impairment and aging among other factors; and (iv) compare ART adherence in HIV-infected patients aged ≤50 years with adherence of younger patients.
Materials and methodsThis study was a cross-sectional study that recruited adult HIV-infected individuals diagnosed according to the protocol of the Brazilian Health Ministry. These patients were receiving care at a Specialized Center (Serviço de Assistência Especializada – SAE) for HIV/AIDS in the city of Pelotas, South Brazil, in 2015. Patients with a previous neurological disease and/or psychotic disorder were excluded from the study.
All patients treated at the SAE were invited to participate in the study, and those who agreed signed the informed consent form. The present study was approved by the Ethics Committee. Participants completed a sociodemographic questionnaire and underwent psychiatric and neurocognitive assessments. The instrument used for psychiatric assessment was the International Neuropsychiatric Interview (MINI – Plus). The neurocognitive assessment was performed using the following instruments: Grooved Pegboard Test, Color Trails Test (CTT) parts 1 and 2, Finger Tapping Test, Montreal Cognitive Assessment, and the International HIV Dementia Scale (IHDS). The cutoff point for the latter was a score of 10 or less. All of the assessments were performed within the SAE premises.
Laboratory data and clinical features were abstracted from the patients’ medical records. Collected information included stage of HIV infection, use of ART, duration of treatment, time of diagnosis, CD4 counts and viral load, and comorbidities. HIV viral load was considered undetectable when less than 50copies/mL.
Adherence to ART was measured by self-reporting and the filling of prescriptions at the pharmacy over the previous three months. The patients who reported to have not forgotten a single dose of medication in the past three days and who regularly filled the ART prescription at the SAE pharmacy over the past three months were considered adherent.
Except for the IHDS, there are no standardized cutoff points for the other neurocognitive assessment instruments in Brazil. Therefore, the scores of the remaining instruments (MoCA, CTT-1 and CTT-2, Finger Tapping Test, and Grooved Pegboard Test) were distributed into quartiles. To obtain higher specificity, the individuals who (i) scored in the upper quartile in at least three of the five instruments used, and (ii) reached the IHDS cutoff point were considered positive for NCI.
Evidence of cognitive impairment was also evaluated by the Instrumental Activities of Daily Living (IADL) scale, as standardized by the Clinical Protocol and Therapeutic Guidelines for the Management of HIV Infection in Adults of the Brazilian Health Ministry.
Sociodemographic and clinical data were subjected to descriptive analysis by calculating frequencies of categorical variables, and the means and standard deviations of continuous variables among patients aged 50 years or more and compared to those aged less than 50 years.
Multiple logistic regression was used to analyze the independent association of age with NCI adjusted for prespecified factors of interest (age, gender, race/ethnicity, education level and cumulative years on ART), in addition to factors with a p-value ≤0.2 in univariate analysis. The analyses were performed using SPSS software (version 21).
A possible independent association of age and adherence to ART was also tested using multiple logistic regression.
ResultsA total of 392 patients participated in the study, 114 of whom were aged 50 years or more with a mean age of 56.7 years, ranging from 50 to 82 years.
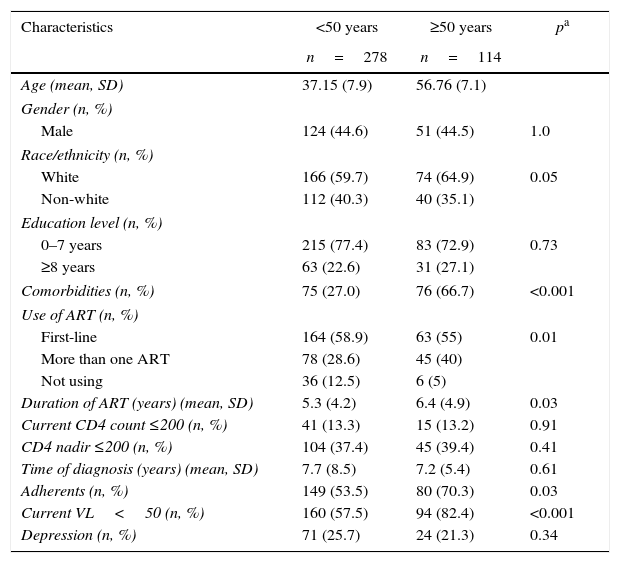
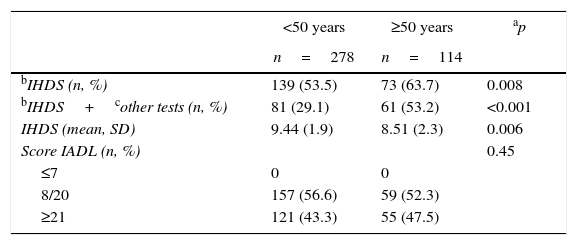
Tables 1 and 2 show a comparison of the characteristics of older and younger patients. The characteristics with significant differences included race/ethnicity, comorbidities (diabetes mellitus, hypertension and dyslipidemia), use of ART, adherence to ART, duration of ART use, currently undetectable viral load, and cognitive impairment (according to the IHDS and the combination of the IHDS and other instruments). Older patients exhibited a higher prevalence of diabetes mellitus, hypertension and dyslipidemia. ART use was more common among patients aged 50 years or more, as was the duration of ART and the rate of patients with undetectable viral loads.
The characteristics of HIV-infected patients aged <50 years and ≥50 years.
| Characteristics | <50 years | ≥50 years | pa |
|---|---|---|---|
| n=278 | n=114 | ||
| Age (mean, SD) | 37.15 (7.9) | 56.76 (7.1) | |
| Gender (n, %) | |||
| Male | 124 (44.6) | 51 (44.5) | 1.0 |
| Race/ethnicity (n, %) | |||
| White | 166 (59.7) | 74 (64.9) | 0.05 |
| Non-white | 112 (40.3) | 40 (35.1) | |
| Education level (n, %) | |||
| 0–7 years | 215 (77.4) | 83 (72.9) | 0.73 |
| ≥8 years | 63 (22.6) | 31 (27.1) | |
| Comorbidities (n, %) | 75 (27.0) | 76 (66.7) | <0.001 |
| Use of ART (n, %) | |||
| First-line | 164 (58.9) | 63 (55) | 0.01 |
| More than one ART | 78 (28.6) | 45 (40) | |
| Not using | 36 (12.5) | 6 (5) | |
| Duration of ART (years) (mean, SD) | 5.3 (4.2) | 6.4 (4.9) | 0.03 |
| Current CD4 count ≤200 (n, %) | 41 (13.3) | 15 (13.2) | 0.91 |
| CD4 nadir ≤200 (n, %) | 104 (37.4) | 45 (39.4) | 0.41 |
| Time of diagnosis (years) (mean, SD) | 7.7 (8.5) | 7.2 (5.4) | 0.61 |
| Adherents (n, %) | 149 (53.5) | 80 (70.3) | 0.03 |
| Current VL<50 (n, %) | 160 (57.5) | 94 (82.4) | <0.001 |
| Depression (n, %) | 71 (25.7) | 24 (21.3) | 0.34 |
CD4 (cells/mm3), cluster of differentiation 4; VL (copies/mL), viral load; ART, antiretroviral therapy; SD, standard deviation; pa values are calculated with X2 test or Fisher exact test.
Neurocognitive impairment and age.
| <50 years | ≥50 years | ap | |
|---|---|---|---|
| n=278 | n=114 | ||
| bIHDS (n, %) | 139 (53.5) | 73 (63.7) | 0.008 |
| bIHDS+cother tests (n, %) | 81 (29.1) | 61 (53.2) | <0.001 |
| IHDS (mean, SD) | 9.44 (1.9) | 8.51 (2.3) | 0.006 |
| Score IADL (n, %) | 0.45 | ||
| ≤7 | 0 | 0 | |
| 8/20 | 157 (56.6) | 59 (52.3) | |
| ≥21 | 121 (43.3) | 55 (47.5) |
IHDS, International HIV Dementia Scale; IADL, instrumental activities of daily living.
There was no difference in the prevalence of depression among the patient groups. The prevalence of NCI according to the IHDS and the combination of the IHDS and other instruments was significantly higher in older patients. The education level was similar in both patient groups.
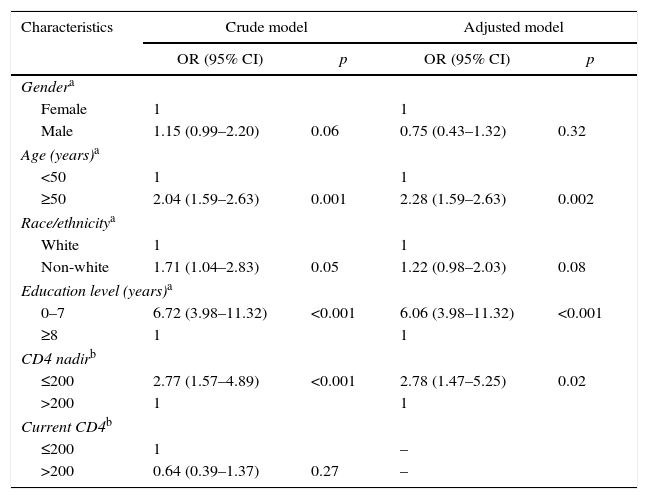
The association of an age ≥50 years with NCI remained significant after adjusting for gender, race/ethnicity, education level, current CD4 count, nadir CD4 count, and duration of ART [OR 2.28 (95% CI: 1.35–3.82, p=0.002)]. In addition to age, other factors shown to be associated with cognitive impairment in the multivariate analysis were education level and nadir CD4 count (Table 3).
Multivariate analysis, cognitive impairment outcome.
| Characteristics | Crude model | Adjusted model | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Gendera | ||||
| Female | 1 | 1 | ||
| Male | 1.15 (0.99–2.20) | 0.06 | 0.75 (0.43–1.32) | 0.32 |
| Age (years)a | ||||
| <50 | 1 | 1 | ||
| ≥50 | 2.04 (1.59–2.63) | 0.001 | 2.28 (1.59–2.63) | 0.002 |
| Race/ethnicitya | ||||
| White | 1 | 1 | ||
| Non-white | 1.71 (1.04–2.83) | 0.05 | 1.22 (0.98–2.03) | 0.08 |
| Education level (years)a | ||||
| 0–7 | 6.72 (3.98–11.32) | <0.001 | 6.06 (3.98–11.32) | <0.001 |
| ≥8 | 1 | 1 | ||
| CD4 nadirb | ||||
| ≤200 | 2.77 (1.57–4.89) | <0.001 | 2.78 (1.47–5.25) | 0.02 |
| >200 | 1 | 1 | ||
| Current CD4b | ||||
| ≤200 | 1 | – | ||
| >200 | 0.64 (0.39–1.37) | 0.27 | – | |
For patients using ART, adherence to treatment was significantly higher among older patients. This association remained significant after adjusting for gender, education level, nadir CD4 count, duration of treatment, depression, and presence of NCI [OR 3.11 (95% CI: 1.67–5.79, p<0.001)].
DiscussionDespite the dramatic reduction in AIDS-related neurological disorders that has occurred since the advent of ART, NCI is still observed in HIV-infected patients and is a significant problem in the management of these patients. Several factors have been associated with cognitive impairment, including advanced age, lower education level, cardiovascular risk factors, CD4 nadir count, the most recent CD4 count and viral load.11,15,19–23
HIV frequently causes encephalopathy. Subcortical structures are mostly affected, leading to NCI, including deficits in attention, learning, memory, information processing speed, and problem solving skills.24 In 2007, the HAND criteria were revised and classified into three conditions: asymptomatic NCI, mild neurocognitive disorder, and HIV-associated dementia (HAD). This nomenclature is referred to as the Frascati criteria.25 Some studies have suggested that advanced age is associated with a higher prevalence of HAD. Valcour et al.26 compared patients older than 50 years with younger patients, finding that the risk of developing HAD was increased by 3.26 (1.32–8.07)-fold in older patients compared to younger patients after adjusting for education level, race, drug addiction, the use of ART, viral load, and CD4 count. In the present study, the risk of exhibiting cognitive impairment was increased by 2.28 (1.35–3.82)-fold in older HIV-infected patients compared to younger patients. The instruments used in the present study primarily allowed for assessing impairment of cognitive functions of the subcortical structures; however, it is not possible to state that this cognitive impairment is secondary to HIV activity on the central nervous system. For the diagnosis of HAND, the causes of cognitive impairment that are traditionally related to cognitive deficit and aging need to be ruled out.
Older patients have a higher risk of exhibiting neurocognitive disorders for causes that are traditionally associated with aging. Cognitive functions become impaired with aging, and advanced age is an important risk factor for most of the neurodegenerative disorders that result in dementia. Recent longitudinal studies have shown that the worst cognitive deficits and neurological impairment in older individuals living with HIV result from (i) pathophysiological mechanisms related to HIV infection of the nervous system, (ii) aging-related factors,27–29 and (iii) a synergistic interaction between HIV and aging.30,31 The study by Seider et al.30 reported significant decline in verbal memory of older HIV-infected patients. The cognitive performance of HIV-infected patients aged 50 and 60 years was similar to that of HIV-negative individuals aged 70 and 80 years, respectively. The present study showed an increased prevalence of cognitive impairment in HIV-infected patients, which is in agreement with the literature.11,12 Furthermore, a higher prevalence of cognitive impairment was found in HIV-infected patients aged 50 years and above compared to younger HIV-infected adults, a result that is in line with other published studies.13–16
The prevalence of cognitive impairment is higher in older individuals in the general population, regardless of the HIV status. The main causes of dementia are Alzheimer's disease and vascular dementia. According to international data, the prevalence of Alzheimer ranges from 4.7 to 8.7% in individuals over 60 years of age,32 and the prevalence of vascular dementia ranges from 1.2 to 4.2% in individuals over 65 years of age.33,34 In addition to these causes of dementia, mild cognitive impairment (MCI) in also present in older individuals, affecting primarily individuals older than 65 years. The prevalence of MCI ranges from 3 to 22%,35,36 depending on the population studied and the criteria used for its definition. Brazilian studies estimate that the prevalence of cognitive and functional impairment in older individuals is between 16 and 19.9%.37–39 In our study, the prevalence of cognitive impairment in HIV-infected patients aged ≥50 years was 53.2%, which is higher than that reported in the literature, especially considering that the mean age of our sample was 57 years and the age considered in studies in non-infected populations is >60 years of age. The extent to which cognitive impairment is (i) secondary to HIV, (ii) secondary to other diseases (e.g., Alzheimer's disease and vascular dementia) or (iii) the result of a synergetic action of HIV with age-related factors that lead to cognitive impairment is not well understood. Comorbidities, such as diabetes, hypertension, and dyslipidemia, were more prevalent in older patients of the present study, as expected. The contribution of chronic HIV infection and the use of ART to these increased prevalence in HIV-infected patients could not be assessed in this study.
Despite the higher prevalence of NCI, adherence to ART was higher in older patients than in younger patients in the present study. Older patients used ART longer and had a higher rate of undetectable viral loads, which is consistent with better adherence. The above-mentioned higher prevalence of adherence among older HIV-infected patients is in agreement with several studies, suggesting that patients of the age-range tended to adhere more promptly to treatment than younger patients.40–43 A recent meta-analysis43 showing that older HIV-infected patients had a lower risk of non-adherence reinforces this finding. Older patients generally have a higher prevalence of factors associated with non-adherence; however, paradoxically, older patients with HIV are more likely to adhere to treatment. Perhaps these patients have been able to maintain ART for a longer period of time due to different factors, such as higher tolerability, and thus are less likely to experience complications that result from severe immunodeficiency and that are responsible for higher short-term mortality.
Among psychiatric disorders, depression is frequently diagnosed in HIV/AIDS patients, with a prevalence ranging between 12% and 66%.19,44–49 In Brazil, studies estimate a prevalence between 32% and 34%.19,46 The study by Passos et al. in the city of Pelotas, Rio Grande do Sul State, Brazil, revealed a high risk of suicide (34.1%).47 Notably, our study found an increased prevalence of depression both in older and younger patients, with no significant difference between the groups. Published studies comparing the prevalence of depression among older and younger HIV-infected patients have reported discordant results.48,49
The present study has some limitations. First, the sample of patients aged 50 years and older was small, reducing the power to detect possible associations of cognitive impairment with factors present among older patients. Second, the instruments for measuring cognitive impairment are not yet fully validated in Brazil, with the exception of the IHDS.50 Nevertheless, the prevalence found in our study was similar to the prevalence reported in other studies.19,21 Another limitation is the evaluation of adherence to ART. Specifically, the use of self-reporting and the short evaluation period (three months) of the filling of ART prescriptions at the pharmacy may have resulted in an overestimation of adherence. However, there was a significant association between adherence and an undetectable viral load, reinforcing the validity of the methodology.
The population of HIV-infected individuals is aging. Therefore, studies aiming at understanding the behavior of HIV infection in this population are necessary. Most of the results that have been published to date have been conducted in high income countries, where the characteristics of the older patient population differ from those of the Brazilian population. The goal of the present study was to describe the characteristics of the patient population aged 50 years and older in a sample from South Brazil. Our results were similar to those from other international studies with respect to cognitive impairment and adherence to treatment.14,15,43 Prospective studies with a larger sample of HIV-infected individuals at advanced age in Brazil are necessary to better understand the characteristics of this population, and the results will allow for improved management of these patients. Furthermore, the easy-to-apply instruments for the diagnosis of HAND need to be validated in Brazil in order to provide a more uniform diagnosis and evaluation of factors related to NCI in our country.
Conflicts of interestThe authors declare no conflicts of interest.