Antimicrobial treatment is often indicated to neutropenic patients. Although renal failure is a common complication of many antibiotics, no information could be found in the literature defining which are the best screening criteria for detecting renal injury. In this paper, the authors aim to assess the progress to renal failure in neutropenic patients on antimicrobial use and to compare different diagnostic criteria of renal failure in association to antimicrobial agents used. This is a cohort study conducted from February to August 2006 at the Hospital das Clínicas of the Universidade Federal de Minas Gerais, which included patients with neutropenia and antimicrobial therapy for the treatment of Healthcare Associated Infections notified by the Hospital Infection Control Committee. Renal injury has ensued in 25% of patients and no statistical difference between distinct criteria for renal injury was observed. Association of greater number of antimicrobials was associated with renal impairment. Time required for renal injury was independent of the antimicrobial regimen used, but mortality among patients with renal injury was higher when compared to those who had preserved renal function.
Neutropenia is a prevalent complication in immunocompromised patients and it is associated with high costs and high morbidity and mortality.1 It is estimated that the incidence of hospitalization for neutropenic patients is 60,000 cases per year in the United States and the average total cost of hospitalization is greater than US$ 20,000 per case.2
Neutropenia increases the risk of infection, but early and empirical use of antibiotics according to international guidelines is a practice that has shown to reduce mortality.1,3 However, antimicrobial agents are associated to side-effects that lead to therapy change or drugs discontinuation.4
One of the most fearful side effects of antibiotics is nephrotoxicity. Unfortunately, guidelines for the management of neutropenic patients include several medications and their associations can cause kidney damage.1,5
It is estimated that, considering critically ill patients, acute renal failure (ARF) is associated with mortality rates exceeding 50%, despite the availability of appropriate care and hemodialysis.6 Among patients who develop chronic kidney disease mortality is also high. The survival rate of dialysis patients along one, two and five years is about 80, 65, and 34%, respectively.7
Although the number of neutropenic patients is increasing and renal injury in this group is a common complication, no information could be found in the literature defining which are the best screening criteria for detecting renal injury.
In this brief communication, the authors aim to assess the progress to renal failure in neutropenic patients on antimicrobial use and compare different diagnostic criteria of renal failure in association to the antimicrobial regimen used.
This is a cohort study conducted at Hospital das Clínicas of Universidade Federal de Minas Gerais (HC/UFMG), from February to August 2006, under the Ethics Committee approval: ETIC 273/09 – UFMG.
Patients with neutropenia and antimicrobial (ATM) agents used for the treatment of Healthcare Associated Infections (HAI) notified by the Hospital Infection Control Committee (HICC) were included. Neutropenia was defined as neutrophil counts ≤500/mm3 or ≤1000/mm3 tending to decline to under 500/mm3 within two days.8
Exclusion criteria were defined as follows: patients younger than four years (considering difference in renal function related to age and different parameters for assessment of creatinine clearance), patients without sequential creatinine measurements suitable for analysis, and patients with high levels of baseline creatinine, according to the established criteria.
Renal insufficiency (RI) was defined when: (a) creatinine increased two times above baseline; (b) creatinine was above 2mg/dL; or (c) creatinine clearance was below 50mL/min; or (d) association of these criteria.
Other variables included in the analysis were: age, weight, underlying disease, hematopoietic stem cells transplantation, number of antimicrobial agents used; association of antimicrobial agents, and time to renal failure.
Renal function was monitored periodically with serum creatinine according to physician indication. Baseline creatinine was considered the first patient's serum creatinine measured. Peak creatinine was considered the highest creatinine level identified along patient monitoring.
Data were analyzed using statistical package Epi-Info version 3.5.2. Descriptive analysis included frequency and percentage, mean or median and standard deviation or range. In the comparative analysis, the χ2 test, Student's t-test or the Mann–Whitney U-test were used, according to studied variables. Statistical significance was defined as p<0.05. The study was approved by the Institutional Review Board (IRB).
A total of 108 patients were eligible for the study. However, 36 patients were excluded. Analysis was carried out with data from 72 patients and median age was 33.5 years (minimum four and maximum 88 years).
Main underlying disease associated with neutropenia was acute myeloid leukemia (26/72=36.1%), followed by chronic myelogenous leukemia (18/72=25%), acute lymphoblastic leukemia (10/72=19.3%), myelodysplasia (7/72=9.7%), lymphoma (6/72=8.3) and other malignancies (5/72=6.9).
Of the 72 patients, 16 (22.2%) underwent allogeneic hematopoietic stem cells transplantation (HSCT) and 16 (22.2%) autologous HSCT. In addition, 23 (31.9%) received nephrotoxic chemotherapy due to underlying disease. There was no statistically significant association for progression to renal failure and the use of these drugs (X2=1.04 and p=0.31). Statistical association was also not observed for main nephrotoxic drugs used in the chemotherapeutic regimen, such as cyclosporine, ifosfamide, cytosin arabinoside e doxorubicin (X2=4.15 and p=0.38).
Eighteen (25%) patients developed RI: in six (8.3%), creatinine clearance was less than 50mL/min; in five (6.9%), creatinine increased two times above baseline; and in only one (1.4%) creatinine was above 2mg/dL. Six patients had a combination of defined criteria for RI. During follow up, only six (33.3%) of 18 patients improved renal function to normal values.
Mean baseline creatinine was 0.8mg/dL (SD±0.3mg/dL), median was 0.8mg/dL and ranged from 0.1 to 1.3mg/dL. Mean creatinine peak level was 1.2mg/dL (SD 0.9mg/dL), median was 0.9mg/dL and ranged from 0.2 to 4.5mg/dL.
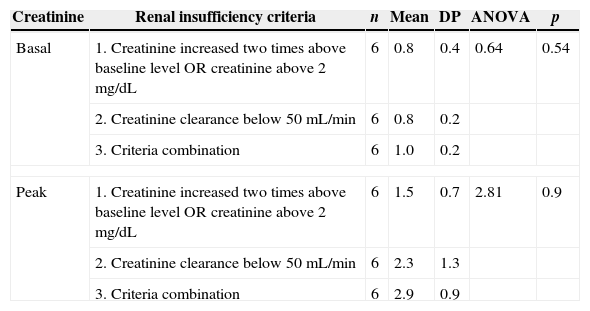
There was no significant difference when baseline creatinine and peak creatinine level were compared in groups of different criteria for RI, but there was a tendency for higher mean values in patients with a combination of criteria (p=0.09). It should be noted that mean values were progressively higher going from criteria 1 to 2 and to 3 (Table 1).
Evaluation of baseline and peak creatinine (mg/dL) levels among neutropenic patients with different criteria for renal failure. HC/UFMG, 2006.
| Creatinine | Renal insufficiency criteria | n | Mean | DP | ANOVA | p |
|---|---|---|---|---|---|---|
| Basal | 1. Creatinine increased two times above baseline level OR creatinine above 2mg/dL | 6 | 0.8 | 0.4 | 0.64 | 0.54 |
| 2. Creatinine clearance below 50mL/min | 6 | 0.8 | 0.2 | |||
| 3. Criteria combination | 6 | 1.0 | 0.2 | |||
| Peak | 1. Creatinine increased two times above baseline level OR creatinine above 2mg/dL | 6 | 1.5 | 0.7 | 2.81 | 0.9 |
| 2. Creatinine clearance below 50mL/min | 6 | 2.3 | 1.3 | |||
| 3. Criteria combination | 6 | 2.9 | 0.9 | |||
An average of 3.9 (SD±2.3) drugs were used, with a median of three drugs, ranging from one to 10 ATM. The majority of patients used two (26.4%) or three (29.2%) ATM. When considering only ATM with significant nephrotoxicity, it was also noticed that great part of patients used two (36.1%) or three (22.2%) of them.
As the patients had several ATM regimens and due to the small sample size in some groups, it was decided to cluster the main groups of ATM for analysis: ceftazidime+aminoglycoside (n=21), ceftazidime+aminoglycoside+vancomycin (n=9), ceftazidime+aminoglycoside+carbapenem+amphotericin (n=7), and any other association of nephrotoxic ATM (n=35).
Patients treated with greater number of ATM agents (ceftazidime+aminoglycoside+vancomycin+carbapenem+amphotericin) had more RI than those who used a different ATM regimen, with statistical significance (p=0.024).
When other ATM regimens were excluded and only the three most frequent associations of ATM used were analyzed, the difference remained statistically significant (p=0.03), revealing that RI was associated with higher number of ATM.
Considering the median time to progression to IR based on any criterion, there was no statistical difference between groups of patients who used different ATM regimens (p=0.33).
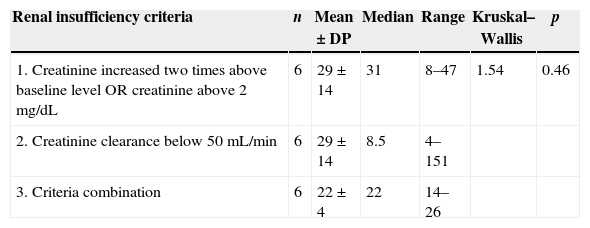
The median time to progression to RI of the 18 patients who had any criterion of IR was 30.7 days (±8.1), with a median of 22 days, ranging from 4 to 151 days. There was no difference when compared the median time to RI between different criteria (p=0.46), according to Table 2.
Median time for progressing (days) to renal failure according to three different criteria in neutropenic patients. HC/UFMG, 2006.
| Renal insufficiency criteria | n | Mean±DP | Median | Range | Kruskal–Wallis | p |
|---|---|---|---|---|---|---|
| 1. Creatinine increased two times above baseline level OR creatinine above 2mg/dL | 6 | 29±14 | 31 | 8–47 | 1.54 | 0.46 |
| 2. Creatinine clearance below 50mL/min | 6 | 29±14 | 8.5 | 4–151 | ||
| 3. Criteria combination | 6 | 22±4 | 22 | 14–26 |
It was observed that patients with RI, regardless of the criteria, were more likely to progress to death (<0.001). Among patients with renal insufficiency (n=18), 50% died during follow-up, percentage significantly higher than the 5.6% mortality of patients without impaired renal function. Patients who died had a mean age of 40.4 years (SD 25.4 years), whereas the mean age among survivors was 30.3 years (SD 20.4 years), difference not significant (p=0.12).
The cause of death was evaluated: eight patients died with sepsis (six with impaired renal function); two with respiratory insufficiency and one with brain death (all of them with impaired renal function), and just one associated to multiple organ failure (without renal impairment during study). Statistical analysis showed no association between cause of death and renal impairment (X2=4.00 and p=0.26).
Co-morbidities were also evaluated in order to identify other causes of RI. Nine patients had co-morbidities (hypertension=4; diabetes mellitus=2 and association with hypertension and diabetes mellitus=3), only one patient with Diabetes mellitus had impaired renal function and there was no statistical difference between groups (X2=3.13 and p=0.37).
The majority of patients in this study (approximately 93%) had a hematological malignancy as underlying disease. This information reflects the profile of patients assisted in the hospital, which is a reference for hematology in the state of Minas Gerais. It also reaffirms the literature, showing that neutropenia is a more frequent complication in the treatment of hematological than solid tumors.9 Another important finding is that 44.4% of the patients in this study underwent bone marrow transplantation, which may reflect the severity of studied sample.
Most patients (75%) maintained preserved renal function, but 25% had RI, which was detected by different criteria or their combination. A study, performed by Zager et al.,10 that included 272 patients with hematologic malignancies who underwent hematopoietic cell transplantation with myeloablative drugs (89% allogeneic transplantation and 11% autologous transplantation) revealed that 53% of patients developed acute kidney injury defined as creatinine increase two times above baseline level.
Values of basal and peak creatinine did not differ statistically in groups of different criteria for RI. This suggests that there was no better criterion of RI for monitoring renal function of neutropenic in this sample. However, there was a trend for higher mean creatinine level in patients with a combination of criteria in the analysis of baseline and peak creatinine. That may reflect a greater severity in patients who have a combination of criteria. One should also be aware that the small sample size might have compromised this analysis.
Another finding was the association of ATM and higher proportion of RI. No analysis was performed to identify if this difference was associated to nephrotoxic drugs (potentiated by ATM association) or if patients who need more drugs and were more severely ill tended to have higher risk of progression to complications.3 The literature shows that combination of nephrotoxic drugs is an independent risk factor for RI, as observed in the study by Oliveira et al.,11 a retrospective cohort analysis of patients admitted to a clinical–surgical ICU (24 beds) in a tertiary-care university hospital over a period of three consecutive years.
There was no difference in median time to onset of renal failure associated to the ATM regimen used. It is important to say that ATM grouped to perform the analysis were not necessarily administered simultaneously, and patients may have used these drugs at different time points during their hospitalization or in sequential therapy for treatment of febrile neutropenia, according to international guidelines.3
Considering renal failure criteria, mean of 30.7 days for the onset of the RI was observed. In the study by Zager et al.,10 in which patients undergoing hematopoietic stem cell transplantation and myeloablative drugs were retrospectively analyzed, this time was 14 days. Lopes and Jorge, in 2011,12 analyzed several studies on the incidence and risk factors for acute kidney injury in patients undergoing transplantation, but not myeloablative used drugs. The authors observed that RI started later, ranging from 22 to 60 days. In the present study, there was no statistical difference between criteria for RI and onset of kidney damage. This finding may reinforce the idea that there is no superior criterium for monitoring renal function in neutropenic patients.
Mortality among neutropenic patients who developed renal failure was significantly higher than among those who preserved the renal function. Several studies have documented an increase in mortality in patients who develop RI after myeloablative and not mieloablative therapy, including short- and long-term mortality.12 In the study by Kersting et al.,13 no difference was observed in mortality among those who developed and not developed any criteria for RI (p=0.002). It was a retrospective study performed on 363 adult patients aged 17–57 years submitted to allogeneic myeloablative stem cell transplantation. After correction for complications with a high mortality, survival of all grades of ARF were comparable, showing that ARF without co-morbid conditions has a good prognosis, and ARF with co-morbid conditions has a poor prognosis. This poor prognosis could be due to the presence of co-morbid conditions rather than to development of ARF itself. Liu et al.14 described a multicenter, retrospective study of acute kidney injury in adult patients with nonmyeloablative hematopoietic stem cell transplantation. The patients with acute kidney injury (AKI) had more than fourfold higher odds of mortality. The odds of mortality in AKI patients were still higher (3.3-fold), even when adjusted for other variables, although it did not reach statistical significance (p=0.054).
The present study did not include an evaluation of risk factors for RI. However, it is known that myeloablative treatment is an independent risk factor for RI, as demonstrated by Parikh et al.15 on a retrospective cohort study from 1997 to 2003 comparing 140 myeloablative and 129 nonmyeloablative patients, and the association of nephrotoxic drugs, as discussed before.11
Although no statistical difference between distinct criteria for RI, association of greater number of antimicrobials determines renal impairment. It was also observed that the time required for RI was independent of the antimicrobial regimen used, but mortality among patients with RI were higher when compared with those who had preserved renal function. It is necessary to emphasize the importance of preservation of renal function, avoiding association of nephotoxic ATM when possible, implying in consequent reduction in morbidity and mortality of this group of patients.
Conflict of interestThe authors declare no conflict of interest.