Tinea capitis is generally considered as the most frequent fungal infection in childhood, as it accounts for approximately 92% of all mycosis in children. The epidemiology of this disease varies widely ranging from antropophillic, zoophilic, and geophillic dermatophytes, as the main causative agent in different geographic areas, depending on several additional factors. Nowadays, the etiology is considered to vary with age, as well with gender, and general health condition. The former reported extraordinary Tinea capitis case reports have been replaced by original articles and researches dealing with progressively changing patterns in etiology and clinical manifestation of the disease. This fact is indicative that under the umbrella of the well-known disease there are facts still hidden for future revelations. Herein, we present two rare cases of Tinea capitis in children, which totally differ from the recently established pattern, in their clinical presentation, as well as in the etiological aspect, as we discuss this potential new etiological pattern of the disease, focusing on our retrospective and clinical observation. Collected data suggest that pathogenic molds should be considered as a potential source of infection in some geographic regions, which require total rationalization of the former therapeutic conception, regarding the molds’ higher antimitotic resistance compared to dermatophytes. Molds-induced Tinea capitis should be also considered in clinically resistant and atypical cases, with further investigations of the antifungal susceptibility of the newest pathogens in the frame of the old disease. Further investigations are still needed to confirm or reject this proposal.
Tinea capitis (TC) is generally considered as the most frequent fungal infection in childhood, as it accounts for approximately 92% of all mycosis in children.1 The ringworm of the scalp is much less common seen in adults, as its incidence beyond childhood is considered as sporadic.1 It is well-known and that TC is caused by dermatophytes, which is a scientific label for a group of three genera of fungi: Microsporum, Epidermophyton and Trichophyton.2 The epidemiology of the disease varies widely ranging from antropophillic, zoophilic, and geophillic dermatophytes; the “leader” in Europe still remains Microsporum canis.3 Nonetheless, the incidence of other antropophillic and zoophilic dermatophytes, Trichophyton tonsurans mainly in the UK, T. tonsurans and Microsporum audouinii in France are progressively increasing.1 Significant interchange of the etiological agents from anthroponoses to zoonoses is observed in contemporary China, as the authors pointed the economic development and urbanization of cities as the main reason for that.4 The earlier human-to-human transmission mode has been replaced by the pets-to-human mode as the source of infection, according to same study.4
Despite the new trends in etiology, contradictory discoveries are also observed in gender distribution of the disease.5 The well-known assertion that TC dominantly affects the female gender, is now replaced by the perception that the gender distribution actually varies depending on the isolated pathogen.1,3 For example, if the TC is caused by Trichophyton genus, there is no significant difference in sexual predilection in children; the frequency of isolation of M. audouinii is up to five-fold higher in males compared to female young patients.5 Nevertheless, M. canis is significantly more frequently seen in male patients, regardless of age.1,3 Significantly higher incidence of the infection is reported among patients of African American origin, as a support of the new trends and changing patterns of the disease.6
The former reported extraordinary TC case reports have been replaced by original articles and researches dealing with progressively changing patterns in etiology and clinical manifestation of the disease. This fact is indicative that under the umbrella of the well-known disease there are facts still hidden for future revelations.
Herein, we present two rare cases of TC in children, which totally differ from the recently established pattern, their clinical presentation, as well as etiological aspects, as we discuss this potential new etiological pattern of the disease focusing on our retrospective and clinical observation.
Case report 1A 9-year-old female patient was presented to the dermatology department with a one-year history of complains of diffuse scalp desquamation and severe itching. Initially, complains were manifested by solitary rounded scaly lesion, which was treated topically with different substances that the patient's mother could not specify. Partial relief had been achieved, but only temporally. Gradually, the desquamation engulfed the entire scalp in the form of a helmet of white scales. The family history was negative for dermatologic disease and neither comorbidities nor co-medication was reported.
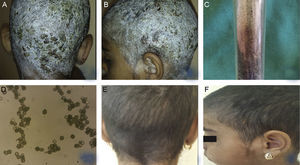
Clinically, the whole scalp was covered with white desquamation with multiple yellowish crusts with areas with yellow exudation, single follicular papules and disseminated exudative vesicles. The neck and ear area was also affected with white desquamation. Hair loss was not observed (Fig. 1A, B).
(A, B) Clinical presentation of TC, caused by Aspergillus niger in a 9-year-old female patient – diffuse white desquamation with multiple yellowish crusts with areas with yellow exudation, single follicular papules, and disseminated exudative vesicles, without hair loss. (C, D) Growth of Aspergillus niger established on mycological examination on Sabouraud agar and direct microscopic evaluation. (E, F) Clinical presentation within the regimen with Terbinafine, dosage 125mg per day, after antibiotic and keratolytic therapy.
Laboratory blood tests were within normal range, except for increased leukocyte count (19.4) and ESR (40mm/h). Microbiological examination of crust exudate revealed Staphylococcus aureus. Direct mycological examination with KOH was negative. Histopathological evaluation excluded psoriasis and did not reveal any specific changes beyond dermatitis. Mycological examination on Sabouraud agar was still pending. Local and systemic antibiotic therapy was initiated with cefuroxime 250mg/5mL in dosage 6mL, bid. Administration of 1mg/mL solution of dimetindene maleate – 20 oral drops three times daily. The scalp was also treated locally with Oleum Acidi Salicilicy 5% under occlusion with rapid decrease of desquamation, but separated alopecic areas with slight scaling was still persistent. The result from the first mycological examination on Sabouraud agar established growth of Aspergillus niger (Fig. 1C, D) The evaluation was repeated and confirmed again. Systemic administration of Terbinafine was initiated at a dosage of 125mg per day, accompanied with topical antimycotic solution. Relief of the clinical symptoms was observed on the 30th day of therapy, which was continued until full improvement and negative mycological examination (Fig. 1E, F).
Case presentation 2A 4-year-old male patient was consulted with dermatologist due to complaints of severe itching of the scalp, accompanied with progressively hair loss, exudation and crust formation of unknown duration. At the time of clinical examination, the patient had already been treated with several courses of systemic antibiotics – oral suspension, topical antibiotics and antiseptics, without any improvement. No family history was available (patient was from an orphanage), but no comorbidities and medication was reported.
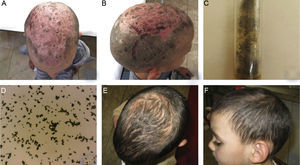
Clinically, hair loss and diffuse desquamation was observed with multiple yellowish and brownish crusts at the area of the vertex, while the underlying skin was very erythematous (Fig. 2A). Single hemorrhagic crusts were observed in right temporal area (Fig. 2B).
(A, B) Clinical presentation of TC, caused by Aspergillus niger in a 4-year-old male patient – hair loss and diffuse desquamation with multiple yellowish and brownish crusts at the area of the vertex, with severe erythema of the underlying skin and hemorrhagic crusts. (C, D) Growth of Aspergillus niger established on mycological examination on Sabouraud agar and direct microscopic evaluation. (E, F) Clinical presentation on the 90th day within the regimen with oral administration of Terbinafine – dosage 75mg per day. Good therapeutic response was observed on 30th day, as hair growth and single areas of effluvium was still observed on the 90th day.
Laboratory tests revealed slightly elevated leukocyte count (14.5g/L) and no other abnormalities. Direct mycological examination with 30% KOH was negative. Mycological examination on Sabouraud agar established growth of A. niger, which was repeated and confirmed (Fig. 2C, D). Oral administration of Terbinafine at a dosage of 75mg per day for three months was initiated. Good therapeutic response was observed on 30th day, as hair growth and single areas of effluvium was still observed on the 90th day (Fig. 2E, F).
DiscussionMycoses in childhood are generally considered as common infections, and Tinea capitis is the commonest clinical type, followed by Tinea corporis and Onychomycosis.7 According to research data reported by Adefemi et al. in 602 children aged 5–16 years, among which 29.9% (180/602) had clinically suspected dermatophytosis lesion (majority of them TC), a dermatophyte was isolated in only 5.0% (30/602) on Sabouraud dextrose agar culture, while nondermatophyte molds represented the majority of isolates, i.e., 15.4% (93/602), without specification of the affected location.7 A similar study recovered nondermatophyte isolates from affected lesions without specifying the location – five isolates were Aspergillus spp. four isolates were Acremonium potronii.2 Although that TC represents the majority of all mycoses in children, as well as the second most common reason for childhood alopecia, after alopecia areata,8 it had been considered till now, that pathogenic molds could not be its causative agent, at least has not received too much attention, contrary to the reported study results, just because the general definition of the disease did not include pathogenic molds.
Recent etiological and epidemiological studies all over the world report more and more contradictory results, which is indicative that not only the main causative agent among separate groups of dermatophytes is different in the different geographical areas throughout the world. Additionally, it points to the increasing alterations in etiological subgroups,1,2 probably as a result of different therapeutic regimens, pharmaceutical progress, and increasing resistance in some species. Besides, the evolutionary reduction of other causative agents, probably due to global alterations in the environment and lifestyle in general has taken place. Nowadays, it is well known that the predominant causative dermatophytic agent varies not only according to the geographic region, but also because of differences in social-economic factors associated with the patient, the age, and even the gender.1–3
Our own results from an 11-year retrospective analysis (2004–2014) of incidence, epidemiology, and etiology of TC in 0–18 year-old children from Bulgaria, revealed surprisingly high incidence of molds – namely A. niger, A. flavus, Penicillium and Rhodotorulla, as etiological agent in 12 of 128 (9.37%) cases of TC. These data prompted us to follow-up the incidence and clinical course of the nondermatophytic TC cases within the following year. A total of six cases, caused by A. niger5 and Penicilium,1 were observed and mycologically examined on Sabouraud dextrose agar for one year. The patients were in 3–9 years of age, with different ethnic origin, different social status, and subsequently different way of living in different areas of the region (rural and urban), while no contact with animals or affected people were reported. In practice, no common risk factor for the occurrence of the disease in these patients was identified.
We decided to evaluate 10 sand-samples from different sandboxes of kindergardens and public playgrounds as putative common risk source of infection. All samples were positive for pathogenic molds, namely Aspergillus spp., Penicillium, Fusarium and Rhodotorulla, while dermatophytes were not established.
All these observations gives rise to the possibility that pathogenic molds could be the new etiologic agent in TC in children, especially in patients with poor living conditions or social deprivation (as in the presented cases). If the earlier human-to-human transmission mode was already replaced by the pets-to-human mode as the source of infection,4 its replacement to environment-to-human mode could not be excluded, as a variety of pathogenic molds can be identified almost everywhere. According to a study by Elias et al., who examined samples from water, water surfaces, air, and other environmental sources in a bone marrow transplantation unit of different hospitals with optimal air precautions, molds and mostly Aspergillus species were recovered in 70% of 398 water samples, in 22% of 1311 swabs from plumbing structures and environmental surfaces, and in 83% of 274 indoor air samples.9 The authors’ discovery entitles them to propose the hypothesis that even the hospital water distribution systems may serve as a potential indoor reservoir of Aspergillus and other molds leading to aerosolization of fungal spores and potential exposure of patients, which could lead to invasive infections.9
The significant changes in the pathogenesis of the fungi in one side, as well as in the host's response to them in another are nowadays natural environment evolution. While evolutionary natural selection is adaptive to the economic growth and environmental changes, some of the former leaders in the etiological structure in TC as Trichophyton schoenleinii are now only sporadically diagnosed, while other, otherwise non-pathogenic species increase their pathogenicity and may be seen as the main causative agent in some atypical form of well-known diseases. Pathogenic molds are not unusually seen as an etiological agent in onychomycosis for example, and although less common, they are capable to cause Tinea corporis. If molds are identified in a sample of TC, they are usually considered as contaminants, just because the definition of the disease does not include them. But if molds can be isolated from the entire surrounding environment, since centuries, as well as they are capable of inducing nail disorders, surely they can be involved in the pathogenesis of TC in some children. Breaking the former stereotype, we raise the question: is it not possible to include pathogenic molds, as the newest pattern in the definition of the epidemiological structures of TC?
ConclusionWe have presented two rare cases of nondermatophyte TC in children, caused by A. niger, treated with antimycotic agents with very good therapeutic results. The progressively changing pattern of the disease suggests that pathogenic molds should be considered as a potential source of infection in some geographic regions, which require total rationalization of the former therapeutic concept, regarding the molds’ higher antimitotic resistance compared with dermatophytes. Molds-induced TC should be also considered in clinically resistant and atypical cases, with further investigations of the antifungal susceptibility of the newest pathogens in the frame of the old disease. Further investigations are still needed to confirm or reject this proposal.
Conflicts of interestThe authors declare no conflicts of interest.