This study verified the occurrence of Bartonella spp. in dogs, cats, wild mammals and their ectoparasites in Petrolina and Lagoa Grande Counties, Pernambuco, located in a semi-arid region in Northeastern Brazil. Anti-Bartonella spp. antibodies were detected by indirect immunofluorescence assay (IFA) in 24.8% of dogs (27/109) and in 15% of cats (6/40). Bartonella sp. DNA was identified by PCR performed on DNA extracted from blood and ectoparasites using primers targeting Bartonella sp. gltA and ribC genes in 100% (9/9) of Pulex irritans from Cerdocyon thous, 57.4% (35/61) of P. irritans from dogs, 2.3% (1/43) of Ctenocephalides felis felis from dogs, 53.3% (24/45) of C. felis felis from cats, and 10% (1/10) of Polyplax spp. from Thrichomys apereoides. DNA sequencing identified Bartonella clarridgeiae and Bartonella henselae in C. felis felis from cats, Bartonella rochalimae in P. irritans from dog and C. thous, and Bartonella vinsoni berkhofii in P. irritans from dog.
Bartonella spp. comprises a group of emerging pathogens that are prevalent in a large variety of vertebrates and cause diversified symptoms and clinical manifestations.1 Transmission predominantly occurs via a vector, and fleas, hematophagous lice, and ticks have confirmed vector competence.2Bartonella DNA has also been isolated from mites.3
Bartonella henselae is the main causal agent of bartonellosis in humans. Although cats are a major reservoir for human infections with these Bartonella species, this agent can infect many mammalian hosts. Thus, rodents and other wild mammals can act as natural reservoirs for numerous species of these bacteria and the presence of the bacteria have been described in these animals on five continents.3–7
In Brazil, the circulation of the agent has been demonstrated in humans, cats, dogs, and wild animals using serological and molecular techniques. However, little information is available about the occurrence of Bartonella in this country, and to date no study has verified the occurrence of Bartonella spp. in the Caatinga biome. Therefore, in the present study we investigated the circulation of Bartonella spp. in domestic mammals (dogs and cats), free-ranging small wild mammals (rodents, marsupials, and canids) and their ectoparasites (fleas, ticks and hematophagous lice) in the Caatinga biome.
Materials and methodsStudy siteThis study was conducted in the Petrolina (9°19′41″S, 40°33′30″W) and Lagoa Grande (8°40′1″S, 40°8′42″W) municipalities, State of Pernambuco, Brazil (Fig. 1). This region has semi-arid tropical weather and is part of the Caatinga biome in Northeastern Brazil.8 The municipalities are located in the region of the São Francisco Valley and represent two distinct areas within a degraded environment.
Sample collectionA total of 77 small mammals were trapped in eight trials from August 2014 to May 2015. Each trial was in a different area, with four in each municipality and two in each season of the year (spring, summer, autumn, and winter). Animals were chemically immobilized as recommended by Mares-Guia.9 Blood samples were collected from these animals via the caudal vein or intra-cardiac puncture. Additionally, ectoparasites were collected from the animals and stored in 1.5mL microtubes containing absolute ethyl alcohol (C2H5OH) at −20°C prior to laboratory analysis. After collection of biological materials, the animals were marked by cutting the hair from the sacral region and were set free in the same point of capture after full recovery of consciousness.
Domicile dogs and cats were analyzed in rural dwellings around each collection area. Information including age, gender, county of origin, tick, flea and/or louse presence, history of ectoparasitism, and access to the forest was obtained. Blood samples were collected from the cephalic vein in tubes containing EDTA, properly identified, and centrifuged at 3000rpm for 15min. Plasma and whole blood were stored in 1.5mL microtubes at −20°C prior to analysis.
Ticks, fleas, and lice were collected and conserved in absolute ethanol and stored at room temperature until identification, according to Linardi and Guimaraes,10 Barros-Battesti, Arzua and Bechara,11 and Pereira et al.,12 and molecular testing.
All procedures followed the ethical standards of animal experimentation established by the Committee on Ethics and Studies and Research at the Federal University of São Francisco Valley – CEDEP/Univasf (protocol number 9/021014) and by the Brazilian Institute of Environment and Renewable Natural Resources - IBAMA (protocol number 45764-1) according to the recommendations and laws regarding the maintenance of animal welfare.
Indirect immunofluorescence assay (IFA)Plasma samples from dogs and cats were subjected to indirect immunofluorescent antibody assay (IFA) (Bion, IL, USA) to detect anti-Bartonella sp. antibodies as recommended by the manufacturer. A cutoff value of 1:64 was used.13
Polymerase chain reaction (PCR)DNA was extracted from whole blood of dogs, cats, and wild animals and from individual ticks using a commercial kit (Promega, Madison, WI, USA) as recommended by the manufacturer. A negative DNA extraction control consisting of 100μL of sterile distilled water was included in each batch of samples. Whole fleas and lice were individually subjected to DNA extraction by boiling at 100°C for 20min.14
All samples were individually processed by PCR using the primers Bhcs.781p (5′-GGG GAC CAG CTC ATG GTG G-3′) and Bhcs.1137n (5′-AAT GCA AAA AGA ACA GTA AAC A-3′), which amplified a 380-bp fragment of the citrate synthase gene (gltA) of Bartonella spp., and BARTON-1 (5′-TAA CCG ATA TTG GTT GTG TTG AAG-3′) and BARTON-2 (5′-TAA AGC TAG AAA GTC TGG CAA CAT AAC G-3′), which amplified a 580-bp fragment of the riboflavin synthase C gene (ribC). The PCR reactions were performed as described previously.15,16 The PCR products were stained with ethidium bromide and visualized by electrophoresis in a 1.5% agarose gel.
PCR products of the expected amplicon size were purified, and their forward and reverse nucleotide sequences were subjected to direct sequencing using the ABI Prism BigDye Terminator v.3.1 cycle sequencing kit (Applied Biosystems, CA, USA). Partial sequences obtained from this study were submitted to BLAST (Basic Local Alignment Search Tool) analysis to determine similarities to other Bartonella species sequences available in the GenBank database.17
Statistical analysisCategorical variables are described as proportions with the respective 95% confidence interval, and compared by the Chi-square test (X2) using the software Epi Info 7.1.
ResultsSeventy-seven small wild mammals were trapped over 2944 trap nights, showing a success rate of 2.6%. The mammals were identified as Rodentia [Thrichomys apereoides (30 specimens), Wiedomys pyrrhorhinus (3), Galea spixii (3), and Calomys expulsus (2)], Marsupialia [Didelphis albiventris (27), Monodelphis domestica (10), and Gracilinanus agilis (1)], and Canid [Cerdocyon thous (1)]. By season, 15 mammals were trapped in winter (September 2014), 17 in spring (November 2014), 20 in summer (January 2015), and 25 in autumn (May 2015).
Hematophagous ectoparasites were found in 51.94% (40/77) of the wild animals. Among Rodentia, 52.63% (20/38) of the animals were infested: T. apereoides by Amblyomma sp. (larvae), Amblyomma auricularium (nymphs), Argasidae (larvae), and Polyplax sp. (adult) and W. pyrrhorhinus by Amblyomma sp (larvae). Among Marsupialia, 50% (19/38) of the animals were infested: D. albiventris by Amblyomma sp. (larvae), A. auricularium (nymphs), Amblyomma dubitatum (nymphs), Argasidae (larvae), Ctenocephalides felis felis (adults), M. domestica by Amblyomma sp. (larvae), A. auricularium (nymphs), and Argasidae (larvae). Among Canidae, 100% (1/1) of the animals were infested: C. thous by Pulex irritans (adults). There was no infestation in C. expulsus, G. spixii, and G. agilis.
Approximately 56% of the dogs (61/109) were infested with Riphicephalus sanguineus adult ticks and 19.26% (21/109) with C. felis felis (11/109) and/or P. irritans (16/109) fleas. Fifteen dogs (13.76%) were parasitized by both ticks and fleas. Around 22% of the cats (9/40) were infested with C. felis felis fleas.
Anti-Bartonella sp. antibodies were detected in 24.77% (27/109) of the dogs and 15% (6/40) of the cats. No significant differences were found among any of the variables analyzed (p>0.05).
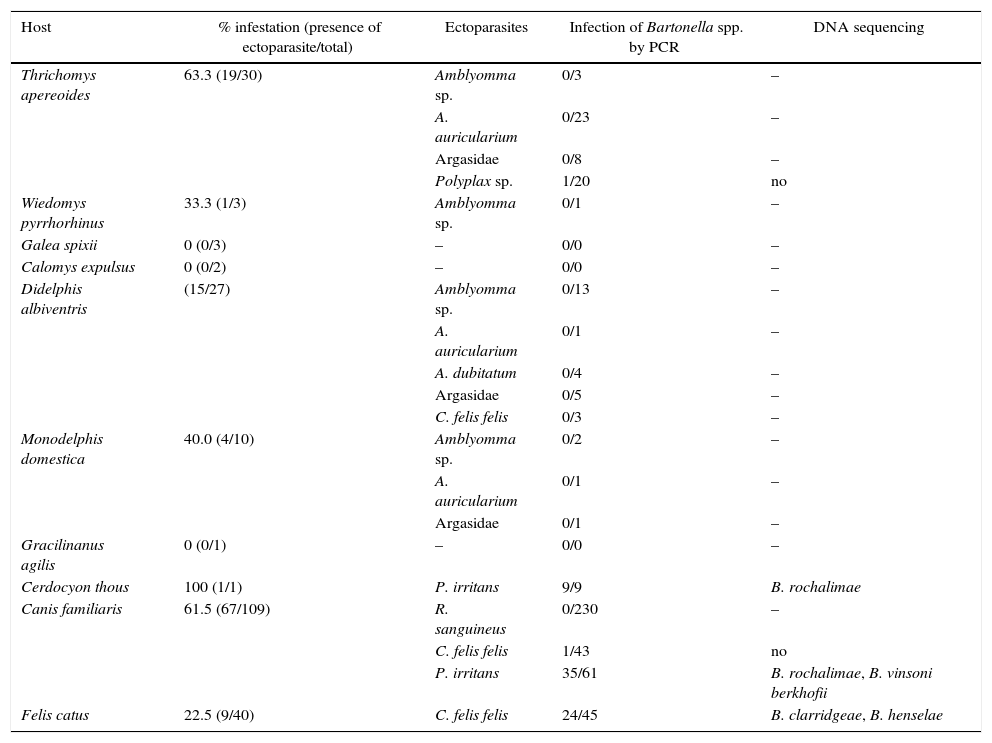
Bartonella sp. DNA was identified in nine (100%) P. irritans fleas from C. thous, one (10%) Polyplax sp. louse from T. apereoides, one (2.32%) C. felis felis and 35 (57.37%) P. irritans from dogs, and 24 (53.33%) C. felis felis from cats. All ticks tested negative by PCR (Table 1).
Bartonella spp. infection in hematophagous ectoparasites in wild and domestic mammals in a semi-arid region of Pernambuco, Brazil.
| Host | % infestation (presence of ectoparasite/total) | Ectoparasites | Infection of Bartonella spp. by PCR | DNA sequencing |
|---|---|---|---|---|
| Thrichomys apereoides | 63.3 (19/30) | Amblyomma sp. | 0/3 | – |
| A. auricularium | 0/23 | – | ||
| Argasidae | 0/8 | – | ||
| Polyplax sp. | 1/20 | no | ||
| Wiedomys pyrrhorhinus | 33.3 (1/3) | Amblyomma sp. | 0/1 | – |
| Galea spixii | 0 (0/3) | – | 0/0 | – |
| Calomys expulsus | 0 (0/2) | – | 0/0 | – |
| Didelphis albiventris | (15/27) | Amblyomma sp. | 0/13 | – |
| A. auricularium | 0/1 | – | ||
| A. dubitatum | 0/4 | – | ||
| Argasidae | 0/5 | – | ||
| C. felis felis | 0/3 | – | ||
| Monodelphis domestica | 40.0 (4/10) | Amblyomma sp. | 0/2 | – |
| A. auricularium | 0/1 | – | ||
| Argasidae | 0/1 | – | ||
| Gracilinanus agilis | 0 (0/1) | – | 0/0 | – |
| Cerdocyon thous | 100 (1/1) | P. irritans | 9/9 | B. rochalimae |
| Canis familiaris | 61.5 (67/109) | R. sanguineus | 0/230 | – |
| C. felis felis | 1/43 | no | ||
| P. irritans | 35/61 | B. rochalimae, B. vinsoni berkhofii | ||
| Felis catus | 22.5 (9/40) | C. felis felis | 24/45 | B. clarridgeae, B. henselae |
One dog was infested simultaneously with positive C. felis felis and P. irritans. Two dogs whose fleas were positive were reactive to the IFA. Fleas from eight (88.88%) cats showed Bartonella sp. DNA, of which two animals had titers of antibodies detectable by the IFA.
Partial sequences of the gltA gene were shown by the BLAST analysis to be 100% identical to the corresponding sequences of Bartonella clarridgeiae (KJ170236), Bartonella henselae (HG965802), Bartonella rochalimae (DQ676484.1) and Bartonella vinsoni berkhofii (AF143445.1) (Table 1).
DiscussionThe study of wildlife allows for an improved understanding of aspects related to the maintenance of various etiological agents in the epidemiological chain of different diseases as well as their sustainability and viability in nature. In this respect, it is important to emphasize studies related to zoonotic diseases due to the massive anthropization suffered by originally sylvan areas.18 In this study, a data survey of fauna ectoparasites was of great relevance due to the vector transmission of Bartonella spp.19
The capture success rate in this study (2.6%) was similar to that obtained by Dantas-Torres et al.20 (1.7%) in the Atlantic Forest fragments in the State of Pernambuco, which is a biome that has suffered severe degradation similar to the Caatinga. However, the success rate was lower than that achieved by Guimarães21 (6.1%) in Serra das Confusões National Park, State of Piauí, which is an environmental preservation area with low human intervention rates. In line with these figures, Fonseca22 compared a non-degraded area to another degraded area in the State of Minas Gerais and obtained success rates of 6.2% and 2.9%, respectively.
Eight different mammalian species were detected, which was equivalent to approximately 5.4% of the total number of naturally occurring mammalian species in the Caatinga (n=148).23 The most frequently caught species was T. apereoides, representing 39% (30/77) of the wild animals in the present study. This species was equivalent to 79% (30/38) of the rodents, which was similar to the 80% reported in Serra das Confusões National Park.21
We obtained a small number of animals infested with fleas in the present study compared to similar studies in both domestic and wild animals.24,25 This result may be related to climatic factors of the study site, which is located in the Drought Polygon region that presents features such as a negative water balance (resulting from an annual rainfall less than 500mm), average insolation of 2800h/year, annual average temperature of approximately 28°C (reaching up to 44.1°C in summer and 39.1°C in winter) and average relative humidity of 50%.26,27 These factors indicate that the site of the present study is a relatively hostile environment for order Siphonaptera, which generally prefers a temperature of 28±1°C and a relative humidity of approximately 75% for its development.28
Although no positive diagnosis was obtained in wild animals blood samples, it was possible to demonstrate the wild cycle of Bartonella spp. in the Caatinga by the presence of bacterial DNA in the fleas and blood-sucking lice that parasitized C. thous and T. apereoides, respectively.
The presence of Bartonella spp. in Polyplax spp. has been previously described in Taiwan.24 The same study also detected DNA of the agent in fleas from wild animals but not in their ticks, in line with the results in our study. More recently, Bartonella vinsonii subsp. arupensis was identified in different species of wild rodents collected in Mato Grosso do Sul, which is the Brazilian state most covered by the Pantanal biome.29
None of the dogs participating in the present study had any apparent sign of disease. Among the seropositive animals (titers≥64), two had a positive diagnosis of Bartonella sp. DNA in their fleas but not in their blood samples. Dogs act as accidental reservoirs of some species of Bartonella. However, asymptomatic dogs rarely show persistent bacteremia.30,31
The present study confirmed the occurrence of Bartonella spp. by detecting antibodies in dogs and cats and the presence of bacterial DNA in ectoparasites collected from domestic and wild animals. These results suggested that the hosts may harbor the microorganism, although Bartonella DNA was not found in any blood samples. Thus, the presence or absence of Bartonella spp. in the host does not necessarily reflect the condition of their fleas.32,33 Negative PCR results in the blood are often indicative of the bacterial DNA concentration in the sample, which may be insufficient for detection by the technique.34,35 Additionally, PCR of blood samples is commonly not successful due to the inhibitory factor hemin.36 Furthermore, Bartonella causes a characteristic, cyclic bacteremia.37
All positive samples tested by PCR targeting the ribC gene were also positive by PCR targeting the gltA gene, but not vice versa. This discrepancy may be due to variability in the sensitivity of the assay depending on the primer used or the primer targeting gltA may have been more comprehensive for the detection of a much wider range of Bartonella species that the primer targeting ribC.15,16,38
The finding that an animal could be PCR-positive and IFA-negative may suggest that the infection is recent, the individual has not produced an IgG antibody level detectable by the test, the animal is immunosuppressed, or the infection is by a Bartonella species that does not present cross-reactivity with B. henselae.39 This possibility may also explain why fleas from an animal are positive for Bartonella DNA when the animal does not have detectable antibodies by IFA as was observed in some animals in this study.
A total of 24.77% (34/109) of the dogs had a suggestive diagnosis for infection by Bartonella spp., and PCR-positive fleas were present on 42.3% (9/21) of the infested dogs. Among these, two dogs were both seropositive and had positive fleas. A lower prevalence was described by Brenner et al.40 in stray dogs in São Paulo (9.3%, 11/118 animals). In Botucatu, state of São Paulo, only 3.5% of 198 dogs were seropositive for Bartonella spp.30 In a study conducted in Peru, a 63% (68/108) seropositivity rate for Bartonella spp. was detected in asymptomatic dogs. The age group was shown to be a risk factor for animals aged less than one year,41 which was in contrast to the present study in which there was no such correlation. Of the 40 cats, six (15%) were positive in the IFA and seven (17.5%) in the PCR test of fleas. One cat was concurrently positive in the IFA and PCR test of fleas. Crissiuma et al.25 reported the occurrence of Bartonella DNA in fleas from 20% (4/20) of infested cats, of which only one had detectable bacterial DNA in the blood.
The serological results in cats in this study are similar to those of Loureiro and Hagiwara,42 who detected 16% positivity for anti-B. henselae antibodies in 200 domicile cats in São Paulo. Among studies conducted with cats in Brazil, the seroreactivity ranged from 1.6% in domestic cats and cats from the Zoonosis Control Center of São Luís, Maranhão, and Cuiabá and Várzea Grande, Mato Grosso43,44 to up to 97% in cats from shelters in Vassouras, Rio de Janeiro,45 using molecular and/or serological methods. The studies were performed in the States of São Paulo [4.3%,46 30%47], Rio de Janeiro [42.5%,25 35.7%,13 and 56.1%48], Mato Grosso [2.2%49] and Rio Grande do Sul [25.5%50].
ConclusionsThe results of the present study confirm for the first time the occurrence of Bartonella in the Caatinga biome and in the semi-arid region and the identification of at least four different species (B. clarridgeiae, B. henselae, B. rochalimae and B. vinsoni berkhofii) in the same geographic region of Northeastern Brazil. This is the first report of Bartonella spp. in Polyplax sp. and Pulex sp. in Brazil. Further investigations are needed to identify the prevalent Bartonella species, to verify the vector competence of these flea species, and to elucidate their epidemiology at the study site.
Conflicts of interestThe authors declare no conflicts of interest.
We thank Ana Isabel A. Santos, Lais F. Santos, Josenilton R. Santos, Ivo W. G. da Silva, and Dália M. R. Machado for their valuable help during the field work and laboratory tests and the Centro de Conservação e Manejo de Fauna (Cema-Fauna) for confirmation of the identification of wild mammalian species. This work was supported by the Brazilian Research Funding Agency (CNPq).