Candida infections account for 80% of all fungal infections in the hospital environment, including bloodstream, urinary tract and surgical site infections. Bloodstream infections are now a major challenge for tertiary hospitals worldwide due to their high prevalence and mortality rates. The incidence of candidemia in tertiary public hospitals in Brazil is approximately 2.5 cases per 1000 hospital admissions. Due to the importance of this infection, the authors provide a review of the diversity of the genus Candida and its clinical relevance, the therapeutic options and discuss the treatment of major infections caused by Candida. Each topography is discussed with regard to epidemiological, clinical and laboratory diagnostic and therapeutic recommendations based on levels of evidence.
Among the fungi of medical interest, yeasts of the genus Candida are of great importance because of the high frequency that they colonize and infect human hosts. Candida species are found in the gastrointestinal tract in 20–80% of healthy adults. Approximately 20–30% of women have vaginal Candida colonization.1 These commensal micro-organisms become pathogenic when there are changes in the mechanisms of host defense or when anatomical barriers secondary to burns are compromised or invasive medical procedures occur. Changes in host defense mechanisms may be due to physiological changes in childhood (prematurity) and aging but are more often associated with degenerative diseases, malignancies, congenital or acquired immunodeficiencies and immunosuppression induced by drugs and medical procedures.2
In the medical community, oral candidiasis and vaginitis caused by Candida account for a significant number of clinical complaints brought to colleagues of different specialties. Candida is the predominant genus among the yeasts of the autochthonous microbiota of the oral cavity and other segments of the gastrointestinal tract. The prevalence of oral cavity colonization by yeasts in normal individuals varies, but most authors report rates of approximately 20–40% in the general population.3 Among the 20 species of Candida of medical importance, Candida albicans is the most prevalent yeast in the oral cavity (accounting for more than 90% of isolates), along with other sites of colonization by this fungus. If there is a disruption of local defense mechanisms, metabolic dysfunction or the presence of diseases associated with immunosuppression, the colonized subject can develop infection and disease.1 Currently, oral candidiasis is the most prevalent opportunistic infection among patients living with AIDS; it is considered a marker of the progression of the immunological deterioration that affects this population. Among treatment-naïve patients infected with human immunodeficiency virus (HIV) or those with no response to highly active anti-retroviral therapy, episodes of oral candidiasis usually become recurrent and may progress to esophagitis.4
Vulvovaginal candidiasis is the second leading cause of infectious leucorrhea. It is responsible for approximately 13 million cases of vaginitis documented annually in North American patients. Surveys reveal that 75% of women experience an episode of vaginal candidiasis during childbearing years, with the estimation that 5% of these women have recurrent episodes.5Candida vulvovaginitis can be sporadic or recurrent, and infections are termed primary or secondary according to the presence or absence of comorbidities associated with this condition. Primary vulvovaginitis is idiopathic and accounts for the vast majority of cases. Secondary vulvovaginitis can have different causes, including hormonal imbalances, metabolic disorders, medications (i.e., antibiotics, contraceptives) and diseases associated with immunosuppression.6
In the hospital environment, Candida infections account for 80% of all fungal infections, including bloodstream, urinary tract and surgical site infections. Pulmonary infections caused by Candida are poorly documented in clinical practice.7 Bloodstream infections are now a major challenge for tertiary hospitals worldwide due to their high prevalence and mortality rates.8 The incidence of candidemia in tertiary public hospitals in Brazil is approximately 2.5 cases per 1000 hospital admissions, a rate considered two to ten times higher than those registered in European and American hospitals and similar to the rates in neighboring countries.9–11 In addition to infection in the bloodstream, urinary candidiasis is common in hospitalized patients. This laboratory finding is controversial, as it may reflect different clinical possibilities that range from a simple contamination of biological material at the time of collection to a colonization of the urinary tract, sepsis or localized invasive disease caused by Candida spp. In most cases, candiduria involves colonization but not urinary infection.12
Diversity of the genus Candida and its clinical relevanceThe genus Candida has become recognized as the nomen conservandum, first at the International Botanical Congress held in Montreal in 1959. This genus consists of approximately 200 species, of which about 20 have been linked to cases of human mycosis.2 Most of the yeasts have no known sexual form, and identification at the species level is obtained by analyzing their micromorphological characteristics and biochemical profiles. Morphological characterization of the majority of isolates of this genus consists of the observation of its capacity to produce blastoconidia, pseudo-hyphae (sometimes true hyphae) and eventually chlamydospores (C. albicans and Candida dubliniensis). In fact, Candida spp. have great genetic diversity and distinct morphological and biochemical characteristics but traditionally have been classified in the same genus.13
Despite the large number of Candida species already described, the main species of clinical interest are C. albicans, Candida parapsilosis, Candida tropicalis, Candida glabrata, Candida krusei, Candida guilliermondii and Candida lusitaniae. However, several cases of superficial and invasive diseases and emerging species of Candida have been described, involving isolates of C. dubliniensis, Candida kefyr, Candida rugosa, Candida famata, Candida utilis, Candida lipolytica, Candida norvegensis, Candida inconspicua, among others.14 Recently, molecular tools have been used in the revision of the taxonomy. These tools are essential for the characterization of some species as agents of emerging infections in the human host, including C. dubliniensis, Candida pseudorugosa, Candida metapsilosis and Candida orthopsilosis; these last two were associated with the complex “psilosis”, formerly characterized as C. parapsilosis genotypes I, II and III.15,16
C. albicans is undoubtedly the most frequently isolated species of superficial and invasive infections at different anatomical sites and in studies worldwide. It is well known as a potentially pathogenic yeast exhibiting pathogenicity and virulence factors including the capacity to adhere to epithelia and various mucous membranes, dimorphism-producing filamentous structures that assist in tissue invasion, significant thermotolerance and the production of enzymes such as proteases and phospholipases.17 This species is naturally sensitive to all systemic antifungal drugs, but cases of acquired resistance to azoles have been reported in patients who have prolonged exposure to these drugs; additionally, few isolates resistant to echnocandins have been also reported.18 Resistance to amphotericin B is considered anecdotal.19
C. dubliniensis has been recognized as a new species whose morphological and biochemical characteristics are very similar to those of C. albicans. Molecular tests are needed to differentiate the two species. This new species was first described in Ireland, where 17–35% of patients with HIV infection have oral colonization or infection with C. dubliniensis.20 In a Brazilian study that evaluated 548 yeast samples stored in a mycology yeast collection, it was determined that 2% of samples originally identified as C. albicans were actually C. dubliniensis.21 This emerging species seems to be less pathogenic than C. albicans, but it has a high probability of developing resistance to azoles.22
C. parapsilosis is an important agent of candidemia and is responsible for 15–30% of candidemias in most series published in Brazil.9,23 In the Northern Hemisphere, the occurrence is higher among children and premature newborns, but C. parapsilosis in Brazil can be found in all age groups.24 The frequency of C. parapsilosis varies between public and private hospitals in Brazil but is prevalent in the public setting.25,26 Characteristically, C. parapsilosis grows in glucose solution, has great capacity to produce “biofilm” and often colonizes the skin of health professionals. Several studies have reported outbreaks of candidemia due to C. parapsilosis associated with the presence of a central venous catheter (CVC) and the use of parenteral nutrition.27 Clinical isolates of this species are usually sensitive to amphotericin B and triazoles.22 However, data generated by the SENTRY – a global candidemia surveillance network – identified some samples of C. parapsilosis resistant to fluconazole.28 High minimum inhibitory concentration (MIC) values for echinocandins have been described against clinical isolates of C. parapsilosis. However, in most cases, these values are still within the range of susceptibility to this class of drugs.29 In comparative clinical trials performed with caspofungin, micafungin and anidulafungin, the three echinocandins available for clinical use, their therapeutic results for infections caused by C. parapsilosis were similar to those obtained with infections caused by C. albicans.30–32 Aside from a clinical study conducted by Moura-Duarte et al. that observed a higher number of cases of persistent candidemia due to C. parapsilosis in patients treated with caspofungin than those treated with amphotericin B, the rate of therapeutic success obtained for infections caused by C. parapsilosis was similar to the rate for C. albicans infections.30 Thus far, in this context, although some authors suggest that there is a possibility of rebound infections caused by C. parapsilosis in patients exposed to echinocandins, data from clinical trials indicate that echinocandins have good efficacy in C. parapsilosis infections.33–35 An important aspect to be considered regarding C. parapsilosis is the recent change in the taxonomy: due to the sequencing of different essential genes of clinical isolates of C. parapsilosis, Tavanti et al. characterized the genetic heterogeneity of this taxon. As a result, “complex psilosis” was reclassified to include three species: C. parapsilosis, C. orthopsilosis and C. metapsilosis.15 The biological differences that may be presented by species within the “complex psilosis” are still not completely understood. However, the isolates from the three species may exhibit differences in patterns of susceptibility to antifungal agents and biofilm production.16,36
C. tropicalis is a potential opportunistic agent when the host is neutropenic and when there is suppression of bacterial flora due to antibiotic use and damage to the gastrointestinal mucosa. C. tropicalis is the second or third most common etiologic agent of candidemia in patients with cancer, particularly leukemia, and less frequently in patients with solid tumors.37 In Brazil, unlike countries in Europe and in the United States, C. tropicalis accounts for a substantial number of documented cases of candidemia in non-neutropenic patients or patients with cancer.9,23,25,26,38,39 Clinical isolates of this species are susceptible to amphotericin B and most of the azoles. However, some authors have documented the occurrence (usually <5%) of isolates resistant to fluconazole. Considering that this species has a strong phenomenon of partial inhibition of growth in in vitro tests (trailing), there is some doubt as to whether the rates of in vitro resistance to fluconazole are overestimated.40
C. glabrata has emerged as an important hospital pathogen, representing the second or third most common species among the agents of candidemia reported in medical centers in Europe and the United States.41 In Latin America, data generated from case series documented until 2005 show that the isolation of C. glabrata candidemia accounted for no more than 5–8% of all episodes of fungemia in public hospitals.9,42 Recently, data from cohorts of private hospitals and medical centers that perform large numbers of organ transplants, where the practice of prophylaxis with fluconazole in high risk patients seems to be more common, indicate that the prevalence of C. glabrata among the causative agents of fungemia reaches more than 10% of the cases.43 Clinical isolates of C. glabrata are less susceptible to fluconazole. Most series documented that 50% of C. glabrata strains have reduced susceptibility to fluconazole and that 10–20% of strains are resistant to this drug.44 Consequently, increases in the rates of colonization/infection by C. glabrata have been observed in different groups of patients exposed to fluconazole.45 In addition to therapeutic issues with azoles in infections associated with C. glabrata, Pfaller et al. observed that isolates of C. glabrata may have lower in vitro susceptibility to amphotericin B and suggested the need for higher doses of polienic for the treatment of invasive infections caused by this agent.46 Another epidemiologic aspect of this pathogen is its high prevalence in elderly patients. In a multicenter study, which evaluated samples of candidemia in 17 medical centers in the state of Iowa, it was observed that C. glabrata is more prevalent in elderly patients and accounted for 25% of all fungemias documented in patients over 65 years.47
C. krusei is an occasional hospital pathogen that is particularly isolated from patients with hematologic malignancies and/or who are undergoing allogeneic hematopoietic stem cell transplant (HSCT).48 Some authors reported increased occurrence of fungemias caused by C. krusei in neutropenic patients exposed to prolonged courses of fluconazole.37 This yeast is naturally resistant to fluconazole, but in most cases, it is sensitive to voriconazole (cross-resistance is uncommon in this species).49
Invasive infections caused by C. guilliermondii are still infrequent, although there are several case reports, especially in patients with cancer.50 Despite the lack of information available in the literature, there are reports of in vitro resistance of clinical samples of C. guilliermondii to amphotericin B, triazoles and echinocandins. The clinical relevance of these in vitro data is still debated; thus, clinical and laboratory monitoring of patients treated with these drugs is recommended to identify treatment failure.51
C. lusitaniae is infrequently a causative agent of invasive disease but has been reported as a candidemia agent in immunocompromised patients. From a total of 86 reported cases of invasive disease by this species, 70 were identified in patients with cancer. Often, clinical isolates of C. lusitaniae have primary or secondary resistance to amphotericin B, but they are very sensitive to all triazoles.52
The epidemiological and therapeutic peculiarities presented by different species of Candida spp. justify the need to identify yeast at the species level when these micro-organisms are associated with systemic diseases. This procedure is fundamental for choosing the best therapeutic approach to be administered to patients. In summary, it is important to note that C. krusei isolates are completely resistant to fluconazole and that, more often than other species (except C. krusei), C. glabrata samples can be resistant to or can require higher doses of azoles for successful treatment. Likewise, higher doses of amphotericin B should be used in the treatment of invasive infections caused by C. krusei and C. glabrata. Finally, clinical isolates of C. lusitaniae may be resistant to amphotericin B.28,46
In this context, it is important to recognize that, for the clinician, the support of mycological diagnostics is essential for the prevention, control and treatment of Candida infections. Full identification of yeast species is necessary; this information is essential not only for the definition of therapeutic choice but also for the control of hospital infection rates at different sites and during the investigation of outbreaks.1 In this sense, it is important to know the wide range of manual and automated commercial systems available that allow rapid and accurate identification of yeasts of clinical interest.53 These guidelines suggest that all medical centers that treat patients at risk for developing invasive fungal infections must have a microbiology laboratory able to identify the main fungal species of medical interest. There is no technical, medical or administrative element that supports the clinical staff of tertiary hospitals for working in medical centers without the basic support of mycological diagnosis.
With regard to susceptibility testing, in view of discussions concerning the existing clinical validation of cutoff points for different therapeutic classes and the difficulty of access to this test for most medical centers in Brazil, it is not possible to recommend its universal use. Therefore, the best scientific evidence available on clinical-laboratory susceptibility tests was generated by in vitro assays performed with Candida species and fluconazole.44,54
Thus, the indication for antifungal susceptibility testing has been evaluated in two different scenarios: during epidemiological investigation and while assisting the clinician at the bedside. In the first scenario, susceptibility tests are needed for surveillance studies of species distribution and for monitoring MICs for different antifungal drugs in several hospital facilities. This allows us to identify and characterize temporal trends and the geographic emergence of pathogens resistant to different drugs, thus supporting a safe indication of empirical therapy.55
While at the bedside, there are four indications for performing susceptibility testing with azole: (a) to evaluate the susceptibility to antifungal agents in patients with hematogenous candidiasis with poor response to the drug in use, information that, along with species identification, is important for guiding a possible change in regimen; (b) to evaluate the susceptibility to fluconazole in a sample of Candida spp. isolated from invasive infections in the event that this triazole was started empirically; (c) to shorten the time therapy started with echinocandin or a lipid formulation of amphotericin B, introducing sequential therapy with oral fluconazole (de-escalation); and (d) for superficial infections with C. glabrata or other Candida strains that may be resistant to fluconazole and to assess the possible in vitro activity of a new oral triazole, such as voriconazole.56
If the medical center decided to make the clinical results of in vitro antifungal susceptibility tests available, testing should be performed by reference laboratories using standardized methodology from regulatory authorities such as the CLSI and EUCAST, or using methods known to be equivalent to these tests, such as E-TEST and Vitek-2.57–60
Therapeutic options for infections caused by Candida spp.During the last decade, the traditional therapeutic compounds, consisting mainly of polienic, imidazole and first-generation triazoles, have been expanded with the development and validation of new systemic antifungal agents. Among the new antifungal agents active against Candida spp. developed in the last decade, we highlight the second-generation triazoles and a novel class of antifungal agents, the echinocandins.
PolienicNystatin and amphotericin B are natural antifungals discovered in the 1950s and obtained from aerobic bacteria (Streptomyces noursey and Streptomyces nodosus, respectively) that have broad-spectrum antifungal activities. In Candida infections, nystatin is reserved for superficial infections due to its topical action. Amphotericin B is indicated for severe forms of invasive candidiasis. The primary mechanism of action is the interaction with steroid components of the cell membranes of eukaryotic cells, leading to rupture. Other mechanisms have been suggested, such as the production of oxygen free radicals by phagocytes in the host. There are different formulations of amphotericin B for intravenous infusion: a deoxycholic acid formulation (amphotericin B deoxycholate or conventional) and lipid formulations (colloidal dispersion, lipid complex and liposomal). The safest lipid formulations in clinical use are amphotericin B lipid complex and liposomal formulation; the latter has lower toxicity and greater tolerability compared to the former formulation.61
Conventional amphotericin B is primarily associated with acute infusion events, including fever, chills, nausea, vomiting, bronchospasm and rash. Fewer side effects are experienced with the lipid complex formulation (two-hour infusion) and particularly with the liposomal formulation (one-hour infusion). The most serious adverse effects are related to the nephrotoxicity of conventional amphotericin B, including the deterioration of renal, cardiac and hematopoietic functions. Of these, renal failure is the most common, occurring in 12–80%, depending on the criteria adopted for renal failure and the population evaluated.62 Among the various alternatives to reduce nephrotoxicity, hydration with 500mL of isotonic saline solution produces better results without compromising effectiveness, but it can be limited in critically ill patients.63 Among the lipid formulations of amphotericin B, the liposomal formulation causes a lower incidence of nephrotoxicity.64,65
Amphotericin B is fungicidal and is active against various Candida species. Secondary resistance is rare. There are data suggesting that amphotericin B MICs for C. glabrata and C. krusei are higher, requiring the use of higher doses of polienic. There is evidence that primary and/or secondary resistance to amphotericin B can occur with clinical isolates of C. lusitaniae.66,67
AzolesThe azoles are a therapeutic class of great clinical utility because of their broad spectrums of action (especially voriconazole and posaconazole), their safety and the availability of oral and intravenous formulations (fluconazole and voriconazole). This therapeutic class can be divided into two groups: the imidazoles and triazoles. The first imidazole with topical action, clotrimazole, was launched in 1960, and it is still being used for superficial candidiasis. In turn, the triazole compounds are subdivided into first-generation (itraconazole and fluconazole) and second-generation (voriconazole and posaconazole) compounds. Isavuconazole, a new second-generation triazole, is still under clinical investigation.68
The azole derivatives are characterized by their selective inhibition of the production of ergosterol, a steroid found in the fungal cell membrane. Their mode of action is the inhibition of fungal 14-α-demethylase, a cytochrome p450-dependent enzyme. Its catalyzing process is essential for the conversion of lanosterol into ergosterol, other actions that can contribute to the antifungal activity have been described, such as inhibition of the yeast transformation into mycelium, the decrease in fungal cell adhesion and the accumulation of steroids that are potentially toxic to fungal cells once the conversion of lanosterol into ergosterol is blocked.69,70 Mechanisms of resistance related to drug efflux, as described with C. glabrata, invariably lead to cross-resistance. Mutations in the gene ERG-11 and changes in the target enzyme 14-α-demethylase, as described with C. krusei and fluconazole, may not cause cross-resistance, as the second-generation triazoles (voriconazole and posaconazole) have higher avidity for the target enzyme.71 Recently, there has been discussion regarding harmonization of the breakpoints of susceptibility to fluconazole, and the MIC value limit for susceptible strains was decreased to 2μg/mL for C. albicans, C. parapsilosis and C. tropicalis.72 Based on this change, higher rates of resistance to fluconazole are expected.73
Because the triazoles are cleared via the hepatic metabolism, many drug interactions are possible.
KetoconazoleKetoconazole was the first imidazole developed for oral therapy of fungal infections. It has a wide spectrum of action against agents of dermatomycoses, endemic mycoses (including paracoccidioidomycosis and histoplasmosis) and isolates of Candida spp. Given its limited efficacy in systemic fungal infections in immunocompromised hosts and its toxicity (hepatotoxicity and depression of steroidogenesis), this drug was replaced by fluconazole and itraconazole in most indications (first-generation triazole).69
ItraconazoleItraconazole is a soluble triazole that is available in capsule form. Its intravenous formulation and oral solution, both in cyclodextrin, are not currently available in Brazil. Although it can be used for infections caused by Candida, the primary indication is for mild to moderate endemic mycoses, such as paracoccidioidomycosis, histoplasmosis, coccidioidomycosis, blastomycosis, chromoblastomycosis, phaeohyphomycosis and sporotrichosis, in addition to dermatomycosis.74,75 Because it is well tolerated in long-term use, and considering its excellent availability in keratinized and subcutaneous tissues, itraconazole can be used in chronic mucocutaneous candidiasis and onychomycosis. It is considered as an alternative drug in cases of oral and vaginal candidiasis. Considering that only the capsule formulation is available in Brazil, itraconazole is not indicated for treatment of hematogenous candidiasis and other invasive forms of mycosis.76
FluconazoleFluconazole is a water-soluble triazole for parenteral (200mg) and oral use (100mg and 150mg) that has antifungal activity against dermatophytes, Cryptococcus neoformans and most Candida spp., except for C. krusei, which has primary resistance, and C. glabrata, which has a lower susceptibility to fluconazole, particularly when isolated from patients with prior exposure to this antifungal. Fluconazole has an excellent safety profile, good absorption in the gastrointestinal tract and distribution in different compartments of the body, including the central nervous system and the eyes. Fluconazole is effective in the treatment of superficial and deep infections by Candida spp., including cases of oroesophageal candidiasis, hematogenous candidiasis and candiduria and its complications.77 Most cases of toxicity to fluconazole are related to drug-induced hepatitis and are often asymptomatic. GI intolerance is not frequent, and leukopenia and thrombocytopenia are rare. Unlike ketoconazole, there is no blockage in hormonal synthesis with fluconazole. The dose should be reduced patients with creatinine clearance <50mL/min.78
VoriconazoleVoriconazole is a triazole available in tablets of 50mg and 200mg and vials of 200mg for intravenous administration whose carrier is cyclodextrin. It has a broader spectrum of action than fluconazole, and it is active against Candida species that include C. glabrata and C. krusei, C. neoformans, Trichosporon sp., Aspergillus spp., Fusarium spp., Scedosporium apiospermum, Histoplasma capsulatum, Blastomyces dermatitidis, Coccidioides immitis and Paracoccidioides brasiliensis. It is not active against Scedosporium prolificans and agents of mucormycosis. The oral formulation has good bioavailability and allows for safe sequential therapy and therapeutic levels in different tissues, including the central nervous system. Dose adjustments are needed in cases of moderate hepatic impairment, and the risks-benefits should be measured in severe forms of liver failure. Renal elimination of the active form is minimal, with no need for dose adjustment when using the oral formulation. However, the use of the intravenous form must be evaluated on a case-by-case basis in patients with creatinine clearance under 50mL/min, as the excipient (cyclodextrin) can be accumulated in patients with renal failure. Regarding safety, the main adverse effects are transient visual disturbances (up to 30% of patients) reversible with discontinuation of the drug, elevations of transaminases and bilirubin, skin reactions and photosensitivity (up to 25%); with use, it is recommended to avoid sun exposure and/or to use sunscreen.79
In the treatment of esophageal candidiasis, voriconazole has clinical efficacy similar to fluconazole. Although its use is most important in invasive aspergillosis, in a study with non-neutropenic patients with candidemia or invasive candidiasis, voriconazole exhibited similar efficacy and less renal toxicity compared to conventional amphotericin B followed by fluconazole.80,81
PosaconazolePosaconazole is a triazole whose chemical structure has been modified from the itraconazole molecule. This azole has a broad antifungal spectrum that acts in vitro and in vivo against isolates of Candida spp., including C. krusei and some isolates of C. glabrata resistant to fluconazole, Aspergillus spp., Fusarium spp., dematiaceous fungi and some agents of mucormycosis. To date, posaconazole is only available in an oral solution that is administered three to four times per day. The absorption can decrease in certain conditions, such as when the patient is receiving a proton pump inhibitor. An oral formulation in tablet form with a single daily administration and improved absorption and an intravenous formulation are under development. While the main indication is prophylaxis of fungal infections in patients with acute myelogenous leukemia and myelodysplastic syndrome receiving remission-inducing therapy as well as transplant recipients of allogeneic hematopoietic stem cells with chronic graft-versus-host disease, the triazole treatment is also indicated as a rescue treatment in several fungal infections, including oropharyngeal candidiasis. However, its unique availability in an oral suspension formulation may be a limitation for patients who are clinically unstable and/or with problems swallowing and absorbing drugs that require oral treatment.82 This drug is not yet available for clinical use in Brazil.
EchinocandinsEchinocandins are a new class of antifungal exclusively for parenteral use that are classified as inhibitors of the enzyme complex 1,3-β-d-glucan synthase, which synthesizes 1,3-β-d-glucan, an essential polysaccharide component of the fungal cell wall. The echinocandins are rapidly fungicidal for Candida species and fungistatic for Aspergillus species.83 Currently, three drugs represent this therapeutic class: caspofungin, micafungin and anidulafungin.
By acting on an exclusive structure of fungal cells (the cell wall), the echinocandins are currently among the most safe and well-tolerated drugs. When present, the adverse effects are mild, such as fever, phlebitis at the infusion site and transient elevation of liver enzymes. In addition to fever, other symptoms mediated by histamine release may rarely occur, including rash, facial swelling, pruritus, sensation of warmth and bronchospasm. Given the small hepatic metabolism of these drugs, few (caspofungin and micafungin) or no drug interactions (anidulafungin) occur with the use of these drugs.83
CaspofunginCaspofungin has been available for clinical use in Brazil for almost a decade. Its formulation is available in vials of 50mg and 70mg. The dose needed for invasive candidiasis is 70mg, followed by 50mg daily. The elimination of the drug occurs by spontaneous hydrolysis and acetylation in the liver; it does not undergo oxidative metabolism by the cytochrome complex P450-dependent enzyme, which explains its low interference with other drugs metabolized in the liver. This antifungal has no renal elimination; therefore, dose adjustment in patients with renal failure is not indicated. In cases of moderate hepatic failure, it is recommended to use a low dosage (35mg/day in adults). There are no clinical data regarding its use in patients with severe hepatic impairment. It has good distribution in different body fluids and tissues, and its concentration is limited in the cerebrospinal fluid, urine and eyes.84 Caspofungin has a large plasma protein binding capacity. This drug should not be used in pregnant women, and there is little clinical information regarding pediatric indications; however, case series suggest that it is an effective and safe choice even in this group.85 Caspofungin has been evaluated in patients with candidemia and/or invasive candidiasis in a randomized trial comparing conventional amphotericin B, which had the same success rate and lower toxicity.30
AnidulafunginThis echinocandin is available in vials of 100mg. Among the few randomized clinical trials available for this drug, two studies have validated its clinical use in esophageal candidiasis and invasive candidiasis/candidemia, both in comparison to fluconazole. In the candidemia/invasive candidiasis study, anidulafungin was one of the few antifungal drugs that yielded the best therapeutic result versus the comparator (fluconazole) in a clinical study involving patients with candidemia.32 Experiences with anidulafungin in the pediatric population, in which the safety and efficacy of caspofungin and micafungin have been demonstrated, are very limited.86,87 This echinocandin has less hepatic metabolism and may be indicated for patients with moderate or severe hepatic impairment without any need for dose adjustment.88
MicafunginThis drug has been sold in vials of 100mg for several years in Japan and has recently begun being sold in the U.S. and Brazil. Among the echinocandins, micafungin is the drug involved in the largest number of phase II and III studies involving patients with candidiasis. In candidemia and invasive candidiasis, studies were compared to liposomal amphotericin B and caspofungina.31,89 Unlike other echinocandins, micafungin does not require a loading dose for treatment initiation.90
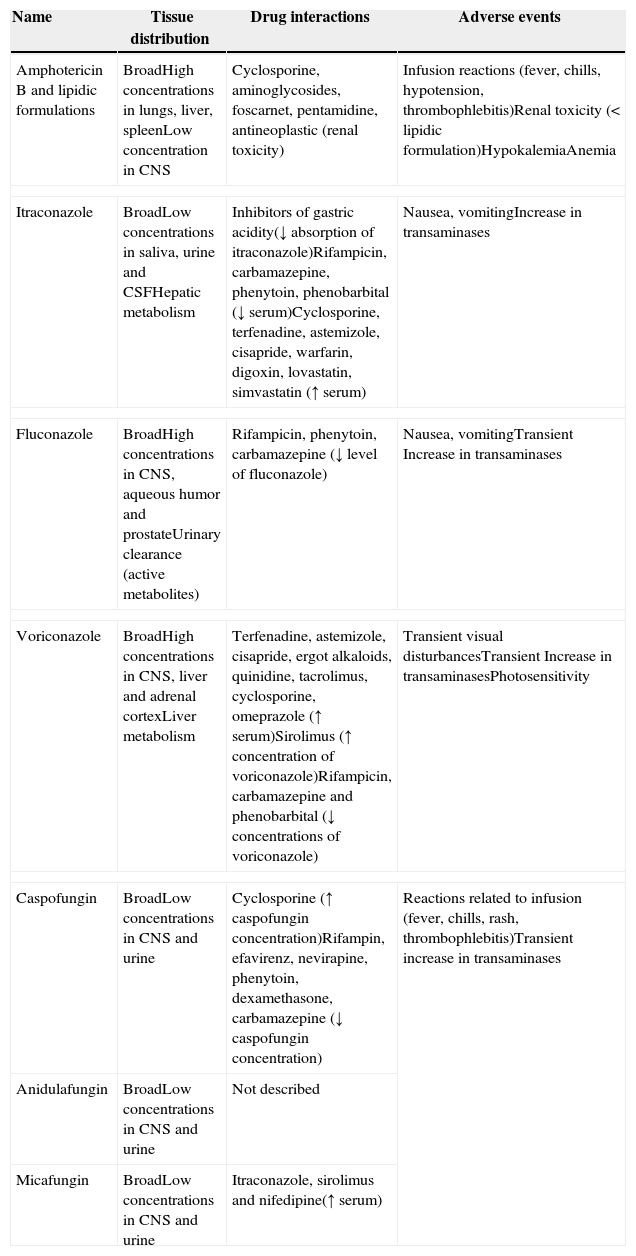
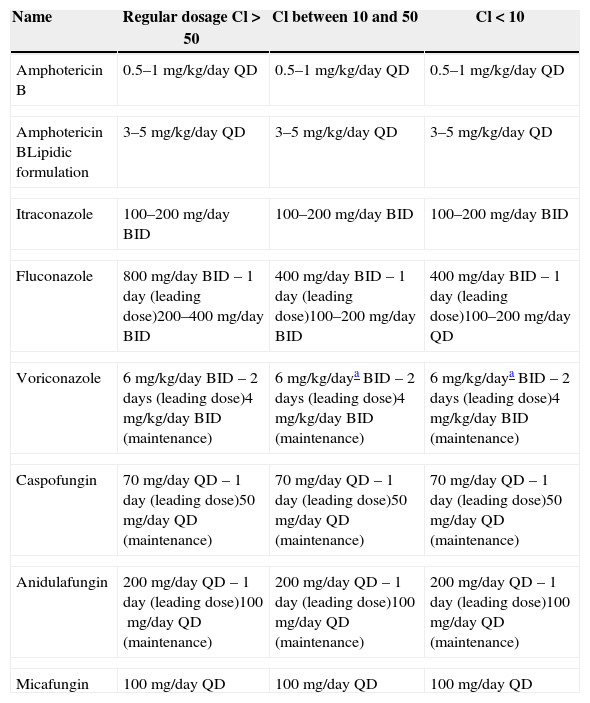
Dosage and drug interactions of antifungalsTables 1 and 2 show the pharmacological aspects and antifungal dosages for systemic use.
Pharmacological aspects of systemic antifungals.
| Name | Tissue distribution | Drug interactions | Adverse events |
|---|---|---|---|
| Amphotericin B and lipidic formulations | BroadHigh concentrations in lungs, liver, spleenLow concentration in CNS | Cyclosporine, aminoglycosides, foscarnet, pentamidine, antineoplastic (renal toxicity) | Infusion reactions (fever, chills, hypotension, thrombophlebitis)Renal toxicity (< lipidic formulation)HypokalemiaAnemia |
| Itraconazole | BroadLow concentrations in saliva, urine and CSFHepatic metabolism | Inhibitors of gastric acidity(↓ absorption of itraconazole)Rifampicin, carbamazepine, phenytoin, phenobarbital (↓ serum)Cyclosporine, terfenadine, astemizole, cisapride, warfarin, digoxin, lovastatin, simvastatin (↑ serum) | Nausea, vomitingIncrease in transaminases |
| Fluconazole | BroadHigh concentrations in CNS, aqueous humor and prostateUrinary clearance (active metabolites) | Rifampicin, phenytoin, carbamazepine (↓ level of fluconazole) | Nausea, vomitingTransient Increase in transaminases |
| Voriconazole | BroadHigh concentrations in CNS, liver and adrenal cortexLiver metabolism | Terfenadine, astemizole, cisapride, ergot alkaloids, quinidine, tacrolimus, cyclosporine, omeprazole (↑ serum)Sirolimus (↑ concentration of voriconazole)Rifampicin, carbamazepine and phenobarbital (↓ concentrations of voriconazole) | Transient visual disturbancesTransient Increase in transaminasesPhotosensitivity |
| Caspofungin | BroadLow concentrations in CNS and urine | Cyclosporine (↑ caspofungin concentration)Rifampin, efavirenz, nevirapine, phenytoin, dexamethasone, carbamazepine (↓ caspofungin concentration) | Reactions related to infusion (fever, chills, rash, thrombophlebitis)Transient increase in transaminases |
| Anidulafungin | BroadLow concentrations in CNS and urine | Not described | |
| Micafungin | BroadLow concentrations in CNS and urine | Itraconazole, sirolimus and nifedipine(↑ serum) | |
Antifungal dosages in humans based on renal function.
| Name | Regular dosage Cl>50 | Cl between 10 and 50 | Cl<10 |
|---|---|---|---|
| Amphotericin B | 0.5–1mg/kg/day QD | 0.5–1mg/kg/day QD | 0.5–1mg/kg/day QD |
| Amphotericin BLipidic formulation | 3–5mg/kg/day QD | 3–5mg/kg/day QD | 3–5mg/kg/day QD |
| Itraconazole | 100–200mg/day BID | 100–200mg/day BID | 100–200mg/day BID |
| Fluconazole | 800mg/day BID – 1 day (leading dose)200–400mg/day BID | 400mg/day BID – 1 day (leading dose)100–200mg/day BID | 400mg/day BID – 1 day (leading dose)100–200mg/day QD |
| Voriconazole | 6mg/kg/day BID – 2 days (leading dose)4mg/kg/day BID (maintenance) | 6mg/kg/daya BID – 2 days (leading dose)4mg/kg/day BID (maintenance) | 6mg/kg/daya BID – 2 days (leading dose)4mg/kg/day BID (maintenance) |
| Caspofungin | 70mg/day QD – 1 day (leading dose)50mg/day QD (maintenance) | 70mg/day QD – 1 day (leading dose)50mg/day QD (maintenance) | 70mg/day QD – 1 day (leading dose)50mg/day QD (maintenance) |
| Anidulafungin | 200mg/day QD – 1 day (leading dose)100mg/day QD (maintenance) | 200mg/day QD – 1 day (leading dose)100mg/day QD (maintenance) | 200mg/day QD – 1 day (leading dose)100mg/day QD (maintenance) |
| Micafungin | 100mg/day QD | 100mg/day QD | 100mg/day QD |
Cl, creatinine clearance (mL/min).
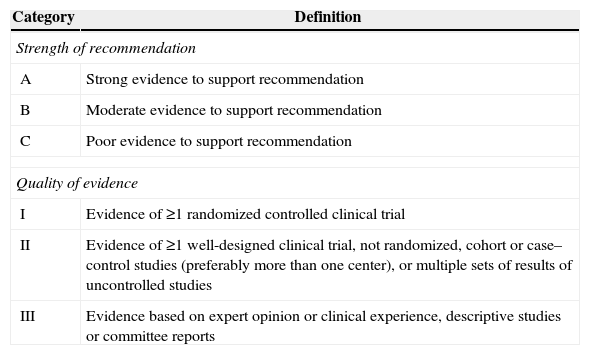
Below, we discuss the treatment of major infections caused by Candida. The recommendations for therapy are indicated for adult patients and were based on levels of evidence according to the strength of the recommendation and the quality of evidence from the American Society of Infectious Diseases, adapted from the Canadian Ministry of Health,91 as shown in Table 3.
Strength of recommendation and quality of evidence.
| Category | Definition |
|---|---|
| Strength of recommendation | |
| A | Strong evidence to support recommendation |
| B | Moderate evidence to support recommendation |
| C | Poor evidence to support recommendation |
| Quality of evidence | |
| I | Evidence of ≥1 randomized controlled clinical trial |
| II | Evidence of ≥1 well-designed clinical trial, not randomized, cohort or case–control studies (preferably more than one center), or multiple sets of results of uncontrolled studies |
| III | Evidence based on expert opinion or clinical experience, descriptive studies or committee reports |
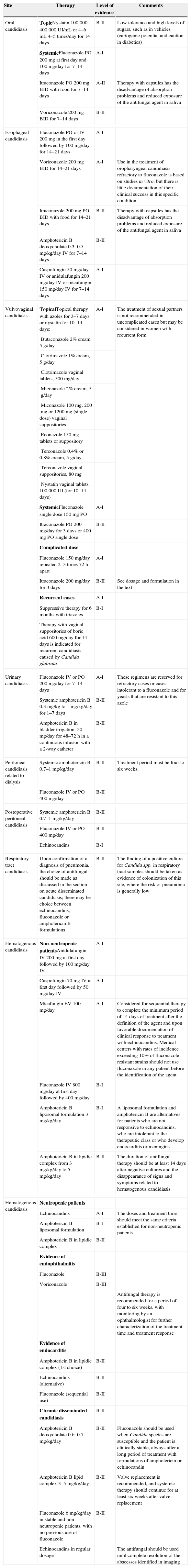
Each topography was discussed with regard to epidemiological, clinical and laboratory diagnostic and therapeutic recommendations. The therapeutic options for treating candidiasis are summarized in Table 4.
Therapeutic regimens for candidiasis.
| Site | Therapy | Level of evidence | Comments |
|---|---|---|---|
| Oral candidiasis | TopicNystatin 100,000–400,000UI/mL or 4–6mL 4–5 times/day for 14 days | B-II | Low tolerance and high levels of sugars, such as in vehicles (cariogenic potential and caution in diabetics) |
| SystemicFluconazole PO 200mg at first day and 100mg/day for 7–14 days | A-I | ||
| Itraconazole PO 200mg BID with food for 7–14 days | A-II | Therapy with capsules has the disadvantage of absorption problems and reduced exposure of the antifungal agent in saliva | |
| Voriconazole 200mg BID for 7–14 days | B-II | ||
| Esophageal candidiasis | Fluconazole PO or IV 200mg in the first day followed by 100mg/day for 14–21 days | A-I | |
| Voriconazole 200mg BID for 14–21 days | A-I | Use in the treatment of oropharyngeal candidiasis refractory to fluconazole is based on studies in vitro, but there is little documentation of their clinical success in this specific condition | |
| Itraconazole 200mg PO BID with food for 14–21 days | B-II | Therapy with capsules has the disadvantage of absorption problems and reduced exposure of the antifungal agent in saliva | |
| Amphotericin B deoxycholate 0.3–0.5mg/kg/day IV for 7–14 days | B-II | ||
| Caspofungin 50mg/day IV or anidulafungin 200mg/day IV or micafungin 150mg/day IV for 7–14 days | A-I | ||
| Vulvovaginal candidiasis | TopicalTopical therapy with azoles for 3–7 days or nystatin for 10–14 days: | A-I | The treatment of sexual partners is not recommended in uncomplicated cases but may be considered in women with recurrent form |
| Butaconazole 2% cream, 5g/day | |||
| Clotrimazole 1% cream, 5g/day | |||
| Clotrimazole vaginal tablets, 500mg/day | |||
| Miconazole 2% cream, 5g/day | |||
| Miconazole 100mg, 200mg or 1200mg (single dose) vaginal suppositories | |||
| Econazole 150mg tablets or suppository | |||
| Terconazole 0.4% or 0.8% cream, 5g/day | |||
| Terconazole vaginal suppositories, 80mg | |||
| Nystatin vaginal tablets, 100,000UI (for 10–14 days) | |||
| SystemicFluconazole single dose 150mg PO | A-I | ||
| Itraconazole PO 200mg/day for 3 days or 400mg PO single dose | B-II | ||
| Complicated dose | |||
| Fluconazole 150mg/day repeated 2–3 times 72h apart | A-I | ||
| Itraconazole 200mg/day for 3 days | B-II | See dosage and formulation in the text | |
| Recurrent cases | A-I | ||
| Suppressive therapy for 6 months with triazoles | B-I | ||
| Therapy with vaginal suppositories of boric acid 600mg/day for 14 days is indicated for recurrent candidiasis caused by Candida glabrata | |||
| Urinary candidiasis | Fluconazole IV or PO 200mg/day for 7–14 days | A-I | These regimens are reserved for refractory cases or cases intolerant to a fluconazole and for yeasts that are resistant to this azole |
| Systemic amphotericin B 0.3mg/kg to 1mg/kg/day for 1–7 days | B-II | ||
| Amphotericin B in bladder irrigation, 50mg/day for 48–72h in a continuous infusion with a 2-way catheter | B-II | ||
| Peritoneal candidiasis related to dialysis | Systemic amphotericin B 0.7–1mg/kg/day | B-II | Treatment period must be four to six weeks. |
| Fluconazole IV or PO 400mg/day | B-II | ||
| Postoperative peritoneal candidiasis | Systemic amphotericin B 0.7–1mg/kg/day | B-II | |
| Fluconazole IV or PO 400mg/day | B-II | ||
| Echinocandins | B-I | ||
| Respiratory tract candidiasis | Upon confirmation of a diagnosis of pneumonia, the choice of antifungal should be made as discussed in the section on acute disseminated candidiasis; there may be choice between echinocandins, fluconazole or amphotericin B formulations | B-II | The finding of a positive culture for Candida spp. in respiratory tract samples should be taken as evidence of colonization of this site, where the risk of pneumonia is generally low |
| Hematogenous candidiasis | Non-neutropenic patientsAnidulafungin IV 200mg at first day followed by 100mg/day IV | A-I | |
| Caspofungin 70mg IV at first day followed by 50mg/day IV | A-I | ||
| Micafungin EV 100mg/day | A-I | Considered for sequential therapy to complete the minimum period of 14 days of treatment after the definition of the agent and upon favorable documentation of clinical response to treatment with echinocandins. Medical centers with rates of incidence exceeding 10% of fluconazole-resistant strains should not use fluconazole in any patient before the identification of the agent | |
| Fluconazole IV 800mg/day at first day followed by 400mg/day | B-I | ||
| Amphotericin B liposomal formulation 3mg/kg/day | B-I | A liposomal formulation and amphotericin B are alternatives for patients who are not responsive to echinocandins, who are intolerant to the therapeutic class or who develop endocarditis or meningitis | |
| Amphotericin B in lipidic complex from 3mg/kg/day to 5mg/kg/day | B-II | The duration of antifungal therapy should be at least 14 days after negative cultures and the disappearance of signs and symptoms related to hematogenous candidiasis | |
| Hematogenous candidiasis | Neutropenic patients | ||
| Echinocandins | A-I | The doses and treatment time should meet the same criteria established for non-neutropenic patients | |
| Amphotericin B liposomal formulation | B-I | ||
| Amphotericin B in lipidic complex | B-II | ||
| Evidence of endophthalmitis | |||
| Fluconazole | B-III | ||
| Voriconazole | B-III | ||
| Antifungal therapy is recommended for a period of four to six weeks, with monitoring by an ophthalmologist for further characterization of the treatment time and treatment response | |||
| Evidence of endocarditis | |||
| Amphotericin B in lipidic complex (1st choice) | B-II | ||
| Echinocandins (alternative) | B-II | ||
| Fluconazole (sequential use) | B-II | ||
| Chronic disseminated candidiasis | B-II | ||
| Amphotericin B deoxycholate 0.6–0.7mg/kg/day | B-II | Fluconazole should be used when Candida species are susceptible and the patient is clinically stable, always after a long period of treatment with formulations of amphotericin or echinocandin | |
| Amphotericin B lipid complex 3–5mg/kg/day | B-II | Valve replacement is recommended, and systemic therapy should continue for at least six weeks after valve replacement | |
| Fluconazole 6mg/kg/day in stable and non-neutropenic patients, with no previous use of fluconazole | B-II | ||
| Echinocandins in regular dosage | The antifungal should be used until complete resolution of the abscesses identified in imaging | ||
Oral candidiasis is considered superficial candidiasis that affects patients with changes in local or systemic immunity, either due to age (premature neonates and the elderly), prosthesis use, exposure to immunosuppressive drugs (chemotherapy, corticosteroids), antibiotics or the presence of diseases such as cancer, diabetes, sarcoidosis, cirrhosis, malnutrition, xerostomy and AIDS.92 In clinical practice, most cases of candidiasis are observed in pediatric patients, who exhibit immaturity of the defense mechanisms of the mucosa, and the elderly, whose defense mechanisms are senescent or even because of the use of dental prostheses.93 The pathological conditions most commonly associated with oral candidiasis in adult patients are AIDS, diabetes and exposure to antibiotics and/or corticosteroids for different conditions. Therefore, all adult patients presenting with oral candidiasis without obvious cause should be investigated for HIV infection.94
C. albicans accounts for approximately 90% of the isolates causing oroesophageal candidiasis, but C. tropicalis, C. krusei, C. glabrata, C. parapsilosis and C. dubliniensis can also be detected.95 In AIDS patients unresponsive to antiretroviral therapy, episodes of oropharyngeal candidiasis become recurrent, requiring prolonged use or repeated cycles of therapy with triazoles. In this scenario, there is an increase in episodes of candidiasis by Candida non-albicans isolates resistant to fluconazole or even in the risk of selecting resistant strains of C. albicans to this drug.96
Clinical and laboratory diagnosisClinical manifestations are varied and depend on the host's immune status and the extent of oral candidiasis. The largest clinical experience of infectious disease is in the form of pseudomembranous candidiasis. The most common symptoms are oral discomfort, burning pain and the presence of removable white plaque under erythematous mucosa. These conditions make feeding difficult, and they can compromise the regularity of oral drug treatments.97 However, other clinical presentations are known. Erythematous candidiasis presents itself as erythematous infiltrate with reduced papillae when present on the tongue. Patients using dental prostheses with oral candidiasis have chronic erythema and discomfort in the region of the prosthesis. Angular cheilitis caused by Candida spp. manifests as discomfort, erythema, and fissures in the angular region of the lips.98
The clinical presentation is usually very characteristic of this condition, particularly when it is pseudomembranous. However, clinical diagnosis should be confirmed by laboratory investigation as follows: (a) by direct mycological examination, with scrapes of lesions in KOH preparations or by Gram staining, where the specimen is analyzed by the presence of fungal elements consistent with Candida spp. and/or (b) by culturing in selective fungal medium (preferably chromogenic medium to identify different species), where the yeast is isolated and the agent is forwarded to complete identification.99
Culture is particularly important in cases of recurrent candidiasis in patients with AIDS, in cases of poor response to conventional therapy or when an injury that is suggestive of candidiasis arises in patients receiving any antifungal drug. In these situations, the identification of the agent species and testing for susceptibility to antifungal agents are necessary recommendations for optimizing a new therapeutic indication in view of the possibility of infection by strains of Candida spp. resistant to one or all triazoles.100,101
Therapeutic recommendationsThe goal of treatment is to eliminate the signs and symptoms of the disease, reduce or eliminate colonization and prevent recurrence.92 Topical therapy is recommended for patients without HIV/AIDS (B-I) and for the initial episodes of cryptococcosis in patients with HIV/AIDS (A-I).
Topical therapy (uncomplicated infection)Nystatin 100,000–400,000IU/mL and 4–6mL four to five times a day for 14 days (B-II) should be administered. Successful treatment depends on the time of contact with the oral mucosa for at least two minutes. It is worth mentioning that this drug has a low tolerance and high sugar content as a vehicle. It also has cariogenic potential and should be used with caution in diabetic patients.98
In the U.S. and Europe, an oral clotrimazole solution is available for use three to five times a day for 14 days (B-II). In these countries, topical therapy is the rule in mild and/or early candidiasis, even in patients with AIDS.102 Unfortunately, in Brazil, clotrimazole is not available in formulations suitable for oral use. In this context, in view of the difficulties in handling nystatin, topical therapy is restricted to only a few patients.
Systemic therapyThe best therapeutic option for systemic candidiasis is oral fluconazole; the other options are considered only in patients unresponsive or intolerant to this drug (A-I).100 Fluconazole 200mg PO in the first day and 100mg/day for 7–14 days (A-I).
In patients with oropharyngeal candidiasis refractory to fluconazole, the options are as follows:
- •
Itraconazole 200mg orally BID with food for 7–14 days (A-II).103,104 Considering that in Brazil we do not have an oral solution, capsules have the disadvantage of impaired absorption and less exposure of the antifungal agent in saliva (B-III).
- •
Voriconazole 200mg BID for 7–14 days. This drug has been validated in comparative clinical trials with fluconazole in patients with esophageal candidiasis (A-I).105 Its use in oral therapy for oropharyngeal candidiasis refractory to fluconazole is based on in vitro studies, but with limited documentation of their clinical success for this specific condition (B-II).
- •
Posaconazole 200mg PO on the first day followed by 100mg orally QD for 13 days for primary therapy (A-I) or 400mg TID for 3 days, followed by 400mg QD for 25 days for refractory cases (B-II). This drug has been validated for this indication in two clinical trials: a randomized comparison with fluconazole and an open study for refractory cases.106,107 Its indication should be reserved for cases of poor response to fluconazole (B-I). This drug is not available in Brazil.
- •
Amphotericin B deoxycholate 0.3–0.5mg/kg/day IV for 7–14 days (B-II).108 This drug should be reserved for cases refractory to fluconazole (B-II).
- •
Caspofungin 50mg/day IV or anidulafungin 200mg/day IV or micafungin 150mg/day IV for 7–14 days. These drugs have been validated in clinical trials comparing fluconazole in patients with esophageal candidiasis (A-I).109–111 The use of these drugs should be reserved for treatment of esophageal candidiasis refractory to fluconazole (B-I).
Given that oral candidiasis is related to the imbalance between the colonizing agent and the local or systemic defense mechanisms, we should try to act toward control of the underlying disease and/or removal of the predisposing conditions. Otherwise, the trend favors chronicity of the process, as it occurs in patients with prostheses and AIDS that is unresponsive to antiretroviral therapy.
Esophageal candidiasisEpidemiological aspectsEsophageal candidiasis is considered a form of semi-invasive candidiasis that primarily affects patients with AIDS, cancer, diabetes, previous esophageal diseases, malnutrition and alcoholism, along with those in therapies using corticosteroids, antibiotics, H2 receptor antagonists and proton-pump inhibitors.92 In clinical practice, most cases of esophageal candidiasis occur in AIDS patients, followed by lower frequencies of diabetics and critically ill patients exposed to multiple antibiotic cycles.99
Clinical and laboratory diagnosisCandida esophagitis can be oligosymptomatic, but its main clinical manifestations include dysphagia, odynophagia and retroesternal burning. In children, nausea, vomiting and dehydration are the main signs. Although the presence of concomitant oral and esophageal candidiasis is common, particularly in AIDS patients, the absence of oral candidiasis does not exclude esophagitis diagnosis. Complications include bleeding, perforation and stenosis.101
In AIDS patients, the diagnosis is usually made based only on clinical data and treatment response. However, taking into account many other opportunistic diseases that affect the esophagus in immunocompromised patients (e.g., herpes and cytomegalovirus), laboratory investigation is mandatory for a definitive diagnosis.94 Endoscopy reports often reveal white plaques that may or may not be accompanied by ulcerated lesions. Apart from the morphological findings, it is recommended to perform a scrap (brush) to obtain a sample for microscopic examination and culturing, in addition to a mucosal biopsy.99
The microscopic examination of fungal elements is performed with a sample obtained by scraping on a slide with KOH or by Gram stain. The culture is performed with a sample obtained by scraping or biopsy. A biopsy should be processed with hematoxylin–eosin staining and silver methenamine (Grocott).99
The definitive diagnosis of esophageal candidiasis is made when, in addition to the clinical and morphological endoscopic findings, we identify fungal elements on microscopic examination and/or observe the presence of fungal elements in tissue, confirming invasion by the pathogen. From an academic point of view, the isolated identification of Candida in culture but no fungal elements by microscopic examination and biopsy may represent colonization of the gastrointestinal tract and not infection.101
Therapeutic recommendationsSystemic therapy is recommended for cases of esophageal candidiasis (B-II). This starts with empirical systemic therapy (A-I) with fluconazole 200mg PO or IV in the first day, followed by 100mg QD for 14–21 days (A-I). When endoscopy is not performed at the time of diagnosis, it should be performed if no improvement occurs within 3–5 days.95
In patients with esophageal candidiasis refractory to fluconazole, the options are as follows:
- •
Voriconazole 200mg BID for 14–21 days. This drug was validated in a comparative clinical trial with fluconazole in patients with esophageal candidiasis (A-I).105 Its use in the treatment of esophageal candidiasis refractory to fluconazole may have a compromised result due to eventual cross-resistance; however, it is a good indication for susceptibility tests, if available (B-II).
- •
Itraconazole 200mg PO BID with food for 14–21 days (A-II).103,104 Given that there is no oral formulation in Brazil and cross-resistance is commonly observed across triazoles, treatment with capsules presents problems with absorption and lesser exposure of the drug to the saliva. These factors can compromise treatment success.
- •
Posaconazole 200mg PO on the first day followed by 100mg PO QD for 13 days for primary therapy (A-I), or 400mg BID for 3 days followed by 400mg QD for 25 days for refractory cases (B-II). This drug was validated for this indication in two clinical trials: one controlled and randomized with fluconazole and another open-label for refractory cases.106,107 Its use for esophageal candidiasis refractory to fluconazole may be compromised by an eventual cross-resistance; however, it is a good indication for susceptibility tests, if available (B-II). This drug is not available in Brazil.
- •
Amphotericin B deoxycholate 0.3–0.5mg/kg/day IV for 7–14 days (B-II).108
- •
Caspofungin 50mg/day IV or anidulafungin 200mg/day IV or micafungin 150mg/day IV for 7–14 days. These drugs were validated in comparative clinical trials with fluconazole in patients with esophageal candidiasis (A-I).109–111
Vaginal candidiasis is highly prevalent in women during their childbearing life; approximately 75% have at least one episode lifelong, and 5–10% can develop a recurrence (defined as at least four episodes of vaginitis by Candida spp. within one year).112
The most frequent predisposing factors for vaginal candidiasis include exposure to high levels of estrogens (birth control, pregnancy and hormone replacement), uncontrolled diabetes mellitus, use of topical and systemic antibiotics and inadequate hygiene habits. Most women with recurrent vaginal candidiasis do not have underlying diseases associated with systemic immunosuppression, and recurrence may be secondary to a deficiency in the local immune response to the agent.113
Vulvovaginal candidiasis is usually classified as complicated or uncomplicated, pending on the severity of the clinical presentation and basic conditions of the host. Uncomplicated forms of vaginitis account for more than 90% of cases and have an excellent response to short oral or topical therapy. Patients with more complicated vaginitis require a prolonged antimycotic therapy.114
C. albicans is the most frequent cause of vaginitis, accounting for approximately 74–95% of cases, followed by C. glabrata in approximately 14.5% of cases. The non-albicans species are more common in recurrent forms and may be found in 10–20% of these patients. C. glabrata is the species most frequently identified in these cases.115,116
Clinical and laboratory diagnosisConsidering that 30% of women may have Candida colonization and there is a wide differential diagnosis for infectious leukorrhea, the diagnosis of C. vulvovaginitis should be based on clinical and laboratory findings.117
Candidiasis involves the vulva and the vaginal lumen, causing intense itching, burning, local discomfort, dysuria, vaginal discharge and dyspareunia. Clinical examination revealed swelling and redness of the vulva and/or vagina, vaginal discharge that looks like milk and, eventually, vulvar carved cracks.118
Clinical diagnosis must be performed by the following tests:117
- •
Direct microscopic examination with the addition of KOH (10%) or Gram stain to search for fungal elements, complemented by evaluation of the vaginal pH (infection usually occurs with a pH between 4 and 4.5);
- •
Culture in specific material. To decrease costs, some authors recommend prompt culture only for complicated or recurrent vulvovaginal candidiasis.
Topical therapy: although most patients prefer oral medications, a meta-analysis comparing 17 studies of uncomplicated vulvovaginal candidiasis revealed similar efficacy between oral and vaginal drugs.119 There is evidence that topically applied azole therapy over a period of 3–7 days is more effective than nystatin, with improvement of symptoms and negative cultures in 80–90% of patients who completed therapy (A-I). Generally, higher concentrations and doses of topical medications are effective over a period of 3 days. Lower doses of the same formulations require more prolonged therapy.102 The options for topical therapy are numerous and include the following:
- •
Butaconazole 2% cream, 5g/day;
- •
Clotrimazole cream 1%, 5g/day;
- •
Clotrimazole vaginal tablets, 500mg/day;
- •
Miconazole 2% cream, 5g/day;
- •
Miconazole, 100mg, 200mg or 1200mg (single dose), vaginal suppositories;
- •
Econazole, 150mg, tablet or suppository;
- •
Terconazole 0.4% or 0.8% cream, 5g/day;
- •
Terconazole, 80mg, vaginal suppositories;
- •
Nystatin, 100,000IU vaginal tablets (10–14 days).
There are formulations containing combination therapy with other agents that will not be commented upon in the text:
- •
Systemic therapy: the use of oral triazoles is a safe and efficient alternative to topical therapy. There is a large amount of clinical experience in treating vulvovaginal candidiasis with fluconazole 150mg QD, single dose (A-I).102 Another option to this drug is itraconazole 200mg QD for 3 days or 400mg single dose (B-II).120 Systemic therapy with triazoles is not indicated in pregnant women. The treatment of sexual partners is not recommended in uncomplicated cases but may be considered in recurrent cases.121
- •
Moderate and severe cases and/or immunocompromised patients: prolonged topical and systemic therapy should be administered to these patients. Topical therapy is recommended for at least 7–14 days using any of the formulations listed above (A-I).102 In case of systemic therapy, the following drugs can be considered:
- º
Fluconazole 150mg/day, repeated two or three times 72h apart (A-I);
- º
Itraconazole 200mg/day for 3 days (B-II).
- º
- •
If the diagnosis of recurrent vulvovaginal candidiasis is made and if there is no identification of or possibility to control or remove the triggering factors, suppressive therapy with triazoles for six months is an effective control measure for recurrent episodes (A-I).122
- •
In such patients, attack therapy can be administered with any of the topical formulations listed above for 7–14 days (A-I) or fluconazole 150mg/day each 72h (days 1, 4 and 7) or until complete symptoms remission; this is the preferred regimen in clinical practice. Once the initial episode is controlled, maintenance therapy with fluconazole 150mg/day once a week for six months is indicated (A-II).122
- •
Although the largest clinical experience of suppressive therapy for recurrent candidiasis was with fluconazole (A-I), there are published trials that suggest maintenance therapy with clotrimazole 500mg suppositories twice a week or itraconazole (200mg PO twice a week or 200mg PO BID monthly) (B-I).123,124
- •
Cases of vulvovaginal candidiasis caused by C. glabrata may not respond to fluconazole. In these cases, vaginal suppositories of boric acid 600mg/day for 14 days are indicated (B-I).125
The term candiduria refers to the growth of Candida spp. in urine cultures collected by appropriate techniques; this finding is not necessarily accompanied by signs and/or symptoms of urinary tract infection (UTI). Candiduria is very frequent among patients exposed to risk factors; up to 20% of hospitalized patients may have candiduria during their hospitalization, particularly intensive care unit (ICU) patients.126,127 This laboratory finding fosters dilemmas regarding its interpretation, as it can represent a simple contamination of the urine collection, candiduria asymptomatic cystitis or pyelonephritis, primary renal candidiasis, ureteropelvic fungus ball or disseminated candidiasis with renal manifestations.
Among hospitalized patients, the factors most often related to the development of candiduria are advanced age, female gender, broad-spectrum antibiotics, the use of corticosteroids and immunosuppressive drugs, the presence of urinary tract abnormalities, diabetes, delayed vesical catheterization, postoperative of major surgery and malignancies.127,128
Series of cases from Brazil confirm that the three most prevalent species isolated from urine in hospitalized patients are C. albicans, C. tropicalis and C. glabrata. These studies measure prevalences ranging from 35.5 to 70% for C. albicans, 4.6–52.5% for C. tropicalis and 7–8.8% for C. glabrata.129–132
Clinical and laboratory diagnosisIn outpatients not exposed to the risk factors mentioned, in most cases, the identification of Candida in urine reflects inadequate collection or processing of the sample and consequent contamination of the culture. In patients exposed to risk factors for UTI by Candida, the finding of candiduria may signify colonization or infection. In these patients, the counting of colonies is highly variable and directly dependent on the methodology used to collect material. Thus, the isolation of Candida in the urine may occur even in the absence of disease, and there is considerable controversy regarding the value of colony counts obtained in culture, a procedure with low specificity and sensitivity in differentiating between patients colonized and infected by this agent.12
Some authors suggest that there is a greater relationship between candiduria and UTI when the colony count in the urine culture reaches values of approximately 10,000–100,000CFU/mL.133,134 However, scores below that can be measured in patients with Candida UTI, particularly in cases of pyelonephritis acquired by the hematogenous route due to systemic candidiasis, in which the kidneys function as filters and may reflect low counts in the urine. In this sense, there is no consensus among authors on the specific cutoff value for the interpretation of quantitative urine cultures for the recognition of patients with infection of the lower UTI or pyelonephritis.135
Therapeutic recommendations- •
The best therapeutic approach for patients with candiduria should be defined on individual basis, considering clinical and epidemiological data to classify each patient into one of the following conditions: (1) no prior risk factors for candiduria, (2) exposure to risk factors but unlikely to be a case of disseminated candidiasis, or (3) exposure to risk factors for candiduria with septicemia without defining etiology and possible/probable systemic dissemination.102,12
- •
The therapeutic approach suggested for these three different scenarios are the following. (1) No prior risk factors for candiduria: in this category, we have patients without underlying diseases who did not undergo catheterization and who have no history of previous use of corticosteroids and antibiotics. They should not receive systemic antifungal agents. It is recommended to request a new collection of material and, if yeasts are found, to investigate the possibility of fungal genital mucositis in the vagina or the glans (C-III).136 (2) Predisposed to candiduria, but unlikely to be disseminated candidiasis: this category includes asymptomatic outpatients or inpatients who underwent catheterization and/or other predisposing factors for candiduria. In these patients, the initial approach is the removal of the predisposing factors with subsequent clinical and laboratory follow-up (C-III). In the vast majority of patients, candiduria resolves after the introduction of these measures. Patients with symptoms of cystitis and with positive urine for yeasts should be treated with antifungal agents (B-III).102,136 (3) Predisposed to candiduria with probable systemic dissemination: critically ill patients with risk factors for systemic fungal infection and who evolve with candiduria and signs of sepsis should be investigated for invasive candidiasis (blood) and should begin the use of systemic antifungal drugs. This means that the patient is not merely colonized (C-III).102
- •
If there are indications for treatment, treatment regimens include the following:
- –
Fluconazole, oral or intravenous dose of 200mg/day for 7–14 days (A-I).137
- –
Amphotericin B, systemic dose of 0.3mg/kg to 1mg/kg/day for 1–7 days (B-II) or amphotericin B, bladder irrigation, 50mg/day for 48–72h with continuous infusion in a two-way tube (B-II). These schemes are reserved for cases refractory infections or those intolerant to fluconazole, along with yeasts resistant to this azole.102,138
- –
In case of suspicion of systemic candidiasis, the patient should be treated according to the recommendations for hematogenous candidiasis.102
- –
Clinical experience with candiduria and echinocandins or voriconazole is restricted; pharmacological data suggest that the urinary concentrations of both antifungals are reduced.139
- –
In the clinical management of patients with candiduria, it is important to consider the removal of the catheterization system, taking into account that this measure may resolves approximately 40% of cases, besides reducing the recurrence of infection (B-I).139 If it is not possible to remove the system, it is at least recommended to change it.140
- –
Peritoneal dialysis is a modality of renal replacement therapy that currently accounts for only 10–20% of dialysis modalities. It can be performed continuously with an oriented procedure performed at home or intermittently, which has been completely abandoned. Among the complications of peritoneal dialysis, infection ranks second place after cardiovascular events, and fungal infections account for 2–14% of peritonitis cases.141 The overall mortality in most series ranges from 10 to 25% of cases, and there are a few reports of up to 50% deaths.142 Among the fungal peritonitis diseases, 80–90% are caused by Candida, particularly isolates of C. albicans, C. parapsilosis and C. tropicalis.143 The risk factors for the occurrence of fungal peritonitis in patients on peritoneal dialysis are not completely known.144 The basic conditions most commonly reported in patients with fungal peritonitis include diabetes, the prior occurrence of peritonitis by other agents and the previous use of antibiotics.145
Clinical and laboratory diagnosisDiagnosis is made through clinical signs and symptoms of peritonitis, which are represented by abdominal pain, distention, and fever associated with clouding of the dialysis fluid, whose cell count increases due to the neutrophil count (>100leukocytes/mm3). Etiologic evidence is obtained by identification of yeasts in bacterioscopic examination of the peritoneal fluid, with growth of Candida spp. in culture.141,145
Therapeutic recommendationsThe guidelines for the treatment of fungal peritonitis are based on case reports and open-label studies of limited groups of patients. Among the key recommendations for the treatment of this complication, the authors suggest that the early removal of the dialysis catheter is essential to the success of the therapy (B-II).146
The largest experience in the treatment of fungal peritonitis is with fluconazole or amphotericin B (B-II). Many authors recommend starting with amphotericin and completing treatment with fluconazole after clinical improvement (B-II).146
Some authors suggest the use of intraperitoneal fluconazole concomitantly with the systemic use of amphotericin B (C-III).147 The treatment period is usually four to six weeks. It is essential to monitor the patient by abdominal ultrasound to rule out collections and to guide the treatment time (B-III).146
There is little reliable information regarding doses of antifungal agents, but the authors suggest the use of 0.7mg/kg to 1mg/kg/day of amphotericin B and 400mg/day of fluconazole.148
If implantation of a new peritoneal catheter is an option, this procedure should be performed with a minimum interval of four to six weeks after the initiation of treatment (C-III). According to recent studies, at least 40% of patients with fungal peritonitis cannot continue with peritoneal dialysis. Another modality for renal replacement therapy is needed.148
Among the new drugs, caspofungin has experienced the most success. It may be considered for patients with poor responses to conventional treatment and can be used at 50–100mg/day with good tolerability (B-II).149 However, in view of the pharmacological similarities and therapeutic success of echinocandins, it is believed that all echinocandins can be used with these conditions (C-III).
Postoperative peritoneal candidiasisEpidemiological aspectsPostoperative peritonitis caused by Candida species occurs with significant frequency in the hospital. The majority of cases are related to episodes of secondary or tertiary peritonitis, when cases of acute abdomen perforated by bacterial peritonitis are subsequently followed by fungal peritonitis. The perforation of the upper digestive tract is more frequently associated with contamination of the peritoneal cavity by Candida compared to the ileum and appendix, occurring in 5–64% of the perforated cases.150
Clinical and laboratory diagnosisThe pathological significance of Candida spp. isolation in the peritoneal fluid and drains of patients undergoing surgery involving manipulation of the gastrointestinal tract is uncertain. The disruption of the anatomical barrier of the gastrointestinal tract can lead to the isolation of transitional agents in the abdominal cavity or contamination of cultures without evolution of the process to properly fungal peritonitis.151 Moreover, a case–control study has isolated Candida spp. in the peritonea of patients who developed perforation of the gastrointestinal tract that caused increased mortality.152
In this context, the interpretation of the identification of Candida in peritoneal fluid should be evaluated on an individual basis, considering the patient's clinical conditions. When Candida is identified in the peritoneal fluid of patients with complicated postoperative recoveries, along with persistent fever and other evidence of peritonitis (for which sepsis is likely from an abdominal source), fungal etiology should be strongly considered. However, in most cases when Candida is isolated in the intraperitoneal fluid cultures of young patients without comorbidities and who have no evidence of systemic infection in postoperative uncomplicated appendicitis, the laboratory finding is generally transitory with no pathological meaning.153
Therapeutic recommendationsAlthough the isolation of Candida in the abdominal cavity is associated with an increase in postoperative complications and mortality, the clinical and laboratory data that should trigger the use of antifungal agents are still a matter of controversy. If there is suspicion of invasive candidiasis, the patient should be treated according to the appropriate therapy for hematogenous candidiasis.153
The most experience in the treatment of peritonitis caused by Candida involves the use of amphotericin B (0.7–1mg/kg/day) or fluconazole (400–800mg/day) (B-II).154 However, the toxicity of amphotericin B and the limited spectrum of fluconazole limit their use in many clinical scenarios.
Taking into account the high rate of success of treating hematogenous candidiasis observed in patients with echinocandins and the large sample of surgical patients in these studies, it is believed that all echinocandins constitute good alternatives in this condition (B-I).149,154
Respiratory tract candidiasisEpidemiological aspectsDespite the controversies, there is a general concept in the literature that Candida pneumonia is an unusual event, particularly among non-neutropenic patients admitted to ICUs. The highest incidences of C. pneumonia are documented among neutropenic patients with hematologic malignancies or patients undergoing lung transplantation.155
In most cases, C. pneumonia is secondary to a hematogenous invasion. In patients undergoing lung transplantation, bronchial anastomosis has been identified as an anatomical site that is potentially more susceptible to colonization and invasion by opportunistic fungi, partly due to the relative ischemia of this region after transplantation. These infections may be complicated by anastomotic dehiscence and subsequent bleeding.156
In ICU patients, especially those undergoing mechanical ventilation, airway colonization by Candida is found with relative frequency, but with no pathological significance. Tracheobronchial colonization by Candida in ICU patients is the result of impairment of local defense mechanisms, the presence of an endotracheal tube, the use of antacids and the exposure to antibiotics, conditions that lead to substantial changes in the microbiota of the oropharynx and the gastrointestinal and respiratory tracts.157
Clinical and laboratory diagnosisThe isolation of Candida in the respiratory tract of critically ill patients, even if obtained by bronchoalveolar lavage, does not allow for the diagnosis of pulmonary candidiasis. In most cases, this finding refers to the colonization and/or contamination of the sample during the procedure. Diagnosis by quantitative culture is not reliable for differentiating colonized patients from those with pneumonia caused by Candida. Thus, the final diagnosis is dependent on lung biopsy with demonstration of the presence of fungal elements in the intima of the parenchyma and supplemented by a culture of tissue fragments with growth of Candida spp.157 In practice, this is rarely a definitive diagnosis.
Therapeutic recommendationsIn general, the identification of positive cultures for Candida spp. in respiratory tract samples should be considered evidence of local colonization whose risk of progression to pneumonia is usually small (B-II).158
Special attention is recommended in the investigation of neutropenic patients, patients with cancer or hematologic malignancies, along with patients undergoing HSCT or lung transplantation (B-II).159–161 When a definitive diagnosis of pneumonia is reached, the antifungal should be chosen as discussed in the section involving acute disseminated candidiasis; there may be a choice between echinocandins, fluconazole or amphotericin B formulations (B-II).149,162
Hematogenous candidiasisEpidemiological aspectsHematogenous candidiasis encompasses a wide spectrum of clinical episodes, including isolates of Candida or cases in which the fungus is present in the bloodstream and spreads to one or more organs of the infected host.1 Considering that most of the data available for hematogenous Candida infection refer to candidemia, this is the term that will be used in these guidelines.
It is believed that the majority of cases of candidemia are acquired via the endogenous route due to the translocation of the pathogen through the gastrointestinal tract, where there is rich colonization by Candida spp. in up to 70% of the general population. Most candidemia events are preceded by colonization by the same species of yeast, which is considered as an independent risk factor for its development. Genotyping methods reveal the similarities between colonizing and infecting strains, confirming the probable endogenous origin of most of the infections caused by these pathogens.163
Any variables causing injury or imbalance in the microbiota of the gastrointestinal mucosa can be facilitators of translocation of Candida spp. to the mesenteric capillaries. Thus, factors that increase intestinal colonization by Candida (i.e., antibiotics, corticosteroids, ileus or intestinal obstruction) or that determine atrophy or intestinal mucosal damage (i.e., prolonged fasting, total parenteral nutrition, hypotension, surgical procedure, mucositis secondary to chemotherapy or radiotherapy) may potentiate the phenomenon of translocation in the gastrointestinal tract.164
Hematogenous infections by Candida spp. can also be acquired exogenously, either by contamination of invasive medical procedures, prostheses or contaminated infusion solutions, such as the colonization of vascular catheters in central positions.24
Case–control studies conducted during the 1980s and 1990s identified numerous risk factors associated with the occurrence of candidemia in hospitalized patients, including: the use of antibiotics, colonization by Candida spp. at different sites, dialysis, major surgery, the use of a CVC in place, chemotherapy, neutropenia, steroid use and parenteral nutrition.165,166
There is a wide geographical variation in the documented etiology patterns of candidemia in different medical centers. In different studies in tertiary hospitals in the public system in Brazil, C. tropicalis and C. parapsilosis are prevalent.42,167 Epidemiology can vary between different institutions; a recent study noted higher incidences of C. glabrata in private hospitals of São Paulo, Rio de Janeiro, Salvador, Belo Horizonte and Curitiba, where the use of fluconazole started in the 1990s. Confirming these data, other series published after 2008 reported rates of candidemia due to C. glabrata and/or C. krusei above 10% in our setting.43,168 These data reinforce the importance of implementing programs for microbiological surveillance of bloodstream infections for the optimization of control strategies and the treatment of these infectious complications.
Clinical and laboratory diagnosisHematogenous candidiasis is an infectious complication that should always be investigated in patients with sepsis after a long period of hospitalization and exposure to risk factors of candidemia, particularly exposure to broad-spectrum antibiotic therapy, invasive medical procedures, immunosuppressive therapy and parenteral nutrition. Brazilian data suggest that 40–50% of these patients are in the ICU at the time of diagnosis. A substantial number of cases have antecedents involving major surgery, particularly with manipulation of gastrointestinal tract.9,42
The study of the natural history of patients with candidemia shows that some episodes of fungemia must be transient and self-limited, particularly in non-neutropenic hosts. However, there are no clinical or laboratory data that allow the clinician to identify with certainty which episodes are only transitory and which will lead to cases of disseminated hematogenous candidiasis with tissue invasion and severe sepsis at the moment of the fungemia diagnosis. Another important aspect to consider is that in some patients, infectious complications documented in the viscera appear weeks or months after a candidemia episode, as occurs in some cases of retinitis, meningitis, or osteomyelitis caused by Candida spp.169,170
These guidelines will discuss in detail the clinical management of three different scenarios of hematogenous candidiasis:
- 1.
Candidemia: isolation of Candida spp. in the bloodstreams of patients without clinical and laboratory evidence of infectious foci in the viscera. In clinical practice, there are few cases for which there is documentation of the involvement of different organs during the episode of candidemia. The most frequent clinical pattern of presentation of candidemia in adults is only in the presence of fever that is unresponsive to antibiotics in patients at risk. The fever may have an insidious onset, without significant involvement of the general condition, or may be accompanied by chills, myalgia, hypotension and tachycardia. Eventually, some patients develop hypothermia and other evidence of sepsis.2
- 2.
Acute disseminated candidiasis: documentation of the presence of concomitant fungemia infection in other organs. When present, the acute spread of candidemia to the organ involves the skin and eye. However, the spread of infection to multiple organs may occur, including cases of pyelonephritis, endocarditis, osteoarticular involvement and involvement of the central nervous system, among others. The appearance of skin lesions can be the first clinical manifestation of invasive disease and is a marker of disease spread. Skin lesions may affect approximately 8% of cases, presenting typically as small nodules or erythematous or purpuric maculopapules, but other morphological features of lesions are described. Systemic candidiasis with skin lesions is particularly frequent in neutropenic patients with candidemia due to C. tropicalis.171 In more recent studies, systematic evaluation of fundoscopy performed by an ophthalmologist suggests that ocular involvement occurs in up to 16% of patients with candidemia, being 2–9% of cases of chorioretinitis and 1% of cases of endophthalmitis.172,173 Symptoms include blurred vision, bulbar scotomas and pain. The ophthalmologic abnormalities are characterized by cotton wool lesions in the retina and vitreous humor, multiple retinal hemorrhages, Roth spots, and uveitis. However, all ocular structures may be affected. When endophthalmitis occurs, therapy is difficult, and the incidence of sequelae is high. The recognition of ocular involvement in patients with candidemia is crucial because the treatment should be administered for a longer period and may eventually require surgery to control the process. The diagnosis should be made early, before the involvement of the vitreous.174 In adults, Candida meningitis usually results from the contamination of a neurosurgical procedure and is rarely documented as a complication of candidemia. However, according to data from autopsy series (which may not represent the general population), patients with sepsis who develop Candida fungal lesions in the central nervous system have died in up to 20% of cases.175 Endocarditis caused by Candida usually occurs as a post-surgical complication of valve replacement surgery and in intravenous drug users, particularly those who use heroin. Endocarditis is rarely reported as a single candidemia complication in a patient who did not undergo cardiac surgery.175 Osteoarticular involvement of candidemia is quite rare but may arise as a late complication (more than one year after the alleged episode of fungemia). Bone involvement is recognized by local pain, fever and radiological findings consistent with osteomyelitis.175
The diagnosis of hematogenous candidiasis in at-risk patients requires careful clinical examination to identify skin lesions and ocular changes consistent with candidemia, in addition to blood cultures.
Blood cultures are a mandatory procedure in any patient with clinical suspicion of systemic infection by Candida, and some care must be taken to optimize the recovery of the agent:
- •
Follow appropriate antisepsis at the puncture site, and remember that the antiseptic must be allowed to act for a few minutes before performing the collection.
- •
It is desirable that blood cultures be performed before use of antimicrobials, or if this is not possible, blood should be harvested in the period preceding the administration of daily doses of drugs.
- •
Blood volume and number of samples are crucial for a good yield of blood cultures; it is recommended that at least two samples per episode of sepsis be collected and that each sample contain at least 20mL of blood (divided into two blood culture bottles per sample).176
- •
Conventional aerobic bottles for automated blood cultures allow the growth of Candida species. However, the performance of aerobic vials may vary between different products. Bactec system vials have lower sensitivity and a longer time for fungal growth than bottles from the BacTAlert system. There are no appreciable differences between these products when using bottles with selective media for fungi.177
- •
It is essential that blood cultures be processed by automated systems, which have better sensitivity and allow for quicker isolation of the agent.
It is important to remember that there is a direct relationship between mortality and the time to onset of treatment of candidemia. Accordingly, every effort should be made for early recognition of patients with hematogenous candidiasis.102
Given the low frequency of the occurrence of visceral lesions in the majority of adult patients with candidemia, the investigation of fungal endocarditis (echocardiography) and lesions in other organs (abdominal imaging) should be reserved for patients who persist with isolation of Candida in blood cultures despite appropriate antifungal therapy or who show signs of clinical deterioration and signs/symptoms suggestive of infection in the abdominal cavity and/or endocarditis. In turn, fundoscopic examination should be performed in all patients with candidemia and visual symptoms. In patients with candidemia but no visual symptoms, it is recommended to perform fundoscopy one week after the initiation of therapy to increase the sensitivity of eye lesion detection.102,174
- •
- 3.
Chronic disseminated candidiasis (CDC): complication documented in patients with neutropenia that develop suppurative lesions predominantly localized in the liver and spleen (but may occur in other organs, particularly the kidney) that manifest after the recovery of neutrophils and capacity of the host inflammatory response. High fever is the most important symptom and occurs in almost all patients; it is associated with anorexia, weight loss, pain in the right hypochondrium, nausea and vomiting. Hepatosplenomegaly is identified in half of the cases. A significant increase in serum alkaline phosphatase, which can be up to ten times the baseline, is the most important laboratory finding for CDC diagnosis in suspected patients with persistent fever after neutrophil recovery.175
A diagnosis can be confirmed with ultrasound, computerized tomography, magnetic resonance imaging or positron emission tomography (PET-CT) of the abdomen, along with findings of swelling of the affected organs and the presence of multiple abscesses in the liver, spleen and/or kidneys. Blood cultures are usually negative, and if a directed biopsy is conducted, necrotic cellular elements can be identified, and fungal elements are absent. In this context, microbiological confirmation of the process is rarely obtained. In most cases, the patient is treated according to the epidemiological and clinical findings, together with the laboratory evidence of CDC represented by abscesses in abdominal imaging and high levels of alkaline phosphatase.175,178 It is important to remember that this situation can occur in infections by other fungi, including yeast (e.g., Trichosporon) and molds (Fusarium, Scedosporium, etc.).
Therapeutic recommendationsThe definition of the best therapeutic strategy to be adopted for patients with hematogenous candidiasis should consider the aspects described below:179
- •
Presence of infectious complications in organs: the occurrence of endophthalmitis, osteomyelitis, endocarditis and CDC are examples of clinical conditions for which antifungal therapy should be extended for periods of four weeks to six months. If prolonged therapy is needed, oral drugs should be chosen.
- •
Severity of the clinical presentation of the case: this issue is controversial, but patients with organ failure are usually treated initially with fast-acting antifungal drugs; fluconazole is generally saved for a second event when there is an initial clinical response and identification of the Candida species.
- •
Determination of Candida species: non-albicans species may exhibit lower susceptibility to fluconazole, requiring dose adjustment or a change in medication.
- •
Risk of renal toxicity while using conventional amphotericin B: the occurrence of acute renal failure in patients in ICUs with renal dysfunction, elderly patients and those receiving other nephrotoxic drugs.
- •
Previous exposure to antifungal prophylaxis regimens and/or empirical therapy: facing a breakthrough infection in a patient exposed to an antifungal agent, a change of therapeutic class is indicated until the involved Candida species and the susceptibility profile of the agent are confirmed.
- •
Presence of an intravascular catheter in a central position: the clinical management of this aspect will be discussed in another section.
- •
The need for surgical removal of the infectious focus: cases of osteomyelitis and endocarditis are examples of clinical situations in which surgical cleaning (or valve replacement) should be considered in the therapeutic management of patients.
We currently have the following drugs available for the treatment of invasive candidiasis: amphotericin B and its formulations, fluconazole, voriconazole and echinocandins.
Candidemia in non-neutropenic patientsIn the last two years, there have been important changes in the epidemiology of candidemia. Several medical centers have reported fungemia rates greater than 10% in adult patients involving species resistant to fluconazole, particularly C. glabrata and C. krusei.43,168,180
Moreover, it is known that the rates of persistent Candida in patients treated with fluconazole are far superior to those of patients treated with drugs most effectives, like echinocandins or formulations of amphotericin B.32,35
In the only study comparing an echinocandin to fluconazole, success rates were significantly higher in patients treated with anidulafungin, even in infections susceptible to fluconazole (C. albicans and C. tropicalis).32 However, for the three echinocandins available in the Brazilian market, there have been substantial price reductions in the daily treatment doses used with this therapeutic class.
A meta-analysis study evaluating therapeutic results of 7 randomized clinical trials performed in 1.915 patients with candidemia/invasive candidiasis involving three therapeutic classes reported that treatment with echinocandins was associated with decreased mortality.181
Given the poor prognosis of this infection in Brazil (50% associated mortality in most series), the high rate of successful clinical and laboratory treatment of candidemia when a broad-spectrum antifungal drug with fungicidal activity is used from the beginning of treatment, and the lower rates of echinocandin toxicity compared to any formulation of amphotericin B, we understand that the best option for initial treatment of this infectious complication is one of the three echinocandins: anidulafungin (A-I), caspofungin (A-I) or micafungin (A-I).30–32 Despite the high MIC values observed with echinocandin when tested against C. parapsilosis, therapeutic results are satisfactory in clinical trials, with no significant differences regarding success rates when compared to infections by other species of Candida.16,34 However, with persistent positive blood cultures for C. parapsilosis, it is recommended that another class of antifungal be started (B-II).
The best use of fluconazole should be considered in sequential therapy to complete a minimum period of 14 days of treatment after determining the etiological agent and upon documentation of a favorable clinical response to treatment with echinocandins (B-I).182
The best use of voriconazole is as an oral sequential therapy in patients infected with strains resistant to fluconazole and susceptible to voriconazole and as a therapeutic approach for patients with central nervous system involvement/endophthalmitis (B-II).81,190 This product should be contraindicated in breakthrough infections after fluconazole therapy and/or invasive infections caused by C. glabrata and C. krusei and in view of the possibility of cross-resistance and limited efficacy in this scenario (B-III).44,81
In view of the renal toxicity of amphotericin B deoxycholate, this drug should be avoided in ICU patients, particularly those exposed to conditions or other nephrotoxic drugs (A-I).183
Fluconazole may be an alternative therapy in clinically stable patients whose infections are considered minor, who were not exposed to regimens of prophylaxis with triazoles, and who are admitted to medical services exhibiting low incidences of infections caused by C. glabrata and C. krusei (B-I).184 Medical centers with rates of incidence exceeding 10% of the fluconazole-resistant strains should not use fluconazole in any patient before the agent is identified (C-III).
Lipid formulations of amphotericin B are an alternative therapy for candidemia, but they have greater renal toxicity than echinocandins. The only lipid formulation in the treatment of Candida assessed in a randomized and comparative study with echinocandin was the liposomal formulation of amphotericin B, indicated at a dose of 3mg/kg/day for the treatment of adults (B-I).31
The lipid complex of amphotericin B has been used in patients with candidemia, but only in open-label non-comparative studies using doses ranging from 3mg/kg/day and 5mg/kg/day (B-II).185
Lipid formulations of amphotericin B are alternatives for patients who: are unresponsive to echinocandins, are intolerant to this therapeutic class, or develop endocarditis or meningitis (B-III).102
Patients with endophthalmitis may not respond to echinocandins, given its low penetration in the eye. In this context, better results are expected with fluconazole or voriconazole (B-II).173
With respect to the time of treatment in all randomized trials conducted with antifungal agents in the last decade, the duration of antifungal therapy was at least 14 days after negative cultures and the disappearance of signs and symptoms of hematogenous candidiasis.102 In this sense, serial blood cultures must be collected until the infection site is negative, and it is recommended to repeat sampling on the third and fifth day after initiation of therapy (at a minimum) to evaluate the success of the microbial treatment (B-III).102
Cases of endocarditis, osteomyelitis, meningitis, or CDC require longer treatment; it is very important to check the availability of antifungal drugs with good bioavailability for oral use (B-II).102
Candidemia in neutropenic patientsPatients with neutropenia should be treated with drugs with a broad-spectrum antifungal drug with fungicidal activity from the beginning of treatment (A-II).186 Given the risk of renal toxicity with conventional amphotericin B, this drug should be avoided in this scenario (B-I).183 Therefore, echinocandins (A-I), liposomal amphotericin B (B-I) and amphotericin B lipid complex (B-II) are considered alternatives.187,188
Randomized trials of candidemia involving caspofungin and micafungin included approximately 10% neutropenic patients. Although there are no data on the performance of anidulafungin in the treatment of candidemia in neutropenic patients, there is no evidence of pre-clinical or clinical order to suggest that echinocandins have differences in their rates of therapeutic success (B-III).
Given the higher incidence of infections caused by C. glabrata and C. krusei in patients with cancer, along with the fact that large percentages of patients with neutropenia are exposed to fluconazole prophylaxis, the recommendation is that the primary treatment of candidemia in patients with cancer and neutropenia not be performed with triazoles (B-II).189 The treatment time must meet the same criteria established for non-neutropenic patients (B-I).102
Infections involving multiple organs or systems must meet the same recommendations given for non-neutropenic patients, along with care for patients referred for C. parapsilosis candidemia treated with echinocandins (B-II).16,34
Patients with evidence of endophthalmitisAll patients with candidemia should have at least one dilated-eye examination performed by an ophthalmologist (A-II).102 Upon diagnosis of endophthalmitis, the drugs better penetrate into the eyeball are fluconazole and voriconazole (B-III).190,191
Early intervention with partial vitrectomy and/or an intravitreal antifungal may be necessary in severe cases (B-III).192 In these cases, we recommend antifungal therapy for a period of four to six weeks, with monitoring by an ophthalmologist for further characterization of the time of treatment and response to therapy (A-III).102
Patients with evidence of endocarditisIn these cases, the greatest experience in the literature involves systemic therapy with an amphotericin B lipid formulation due to the possibility of using high dosages (B-II).193 Alternatives include echinocandin (B-II) and fluconazole, which should be used when the Candida species is susceptible and the patient is clinically stable (B-III).194–196 Although amphotericin B is considered an effective alternative, in view of its potential toxicity and the treatment duration required, its use should be avoided (B-II).183 A valve replacement is recommended, and systemic therapy should continue for at least six weeks after valve replacement (B-III).197
Patients with chronic disseminated candidiasisGiven the low incidence of this complication, there are no comparative data regarding efficacy and tolerability between the different antifungals.
The treatment of this condition is always long, so starts with a broad-spectrum fungicidal drug until clinical improvement is achieved, which is followed by oral fluconazole for three to six months (A-III).198 The antifungal should be used until complete resolution of the abscess, as detected by imaging (A-III).198
The greatest experience in treating patients with CDC involves amphotericin B formulations (B-II).198 In case of infection control and as long as the patient continues receiving antifungal drugs, there are no contraindications for starting a new cycle of chemotherapy or for the transplantation of hematopoietic stem cells (B-II).199,200
The therapeutic options are amphotericin B deoxycholate at a dose of 0.6–0.7mg/kg/day (B-II); an amphotericin B lipid formulation at a dose of 3–5mg/kg/day (B-II)201; fluconazole 6mg/kg/day in stable and non-neutropenic patients who have not previously used fluconazole (B-II)202,203 and echinocandins in the usual doses (B-II).204
As manifestations of CRC result from an exaggerated inflammatory response, the use of corticosteroids as an adjuvant therapy may be useful. In a series of cases, patients who received corticosteroids experienced rapid resolution of fever and general symptoms (B-II).178
Management of a central venous catheterMost patients with candidemia have one venous catheter in the central position upon diagnosis. The reason for removal of the CVC in patients with candidemia is the fact that Candida can colonize the CVC, producing a biofilm, and lack of removal may result in persistence of a focus of infection. Several retrospective studies have analyzed the impact of CVC removal on outcomes such as duration of candidemia and mortality; the majority of these studies reported lower mortality rates when the CVC was removed.205–210 These studies form the basis for recommendations to remove the CVC in the guidelines of candidemia management published in recent years.102 However, these studies have several limitations, including the lack of multivariate analysis, in particular severity scores, the inclusion of early deaths and, most importantly, the absence of setting a time for the withdrawal of the CVC.
A recently published study analyzed 842 episodes of candidemia in adults and conducted a sub-analysis of two randomized trials of candidemia treatment with echinocandins (caspofungin or micafungin) or liposomal amphotericin B. We investigated the effect of early removal (24 or 48h after initiation of candidemia treatment) in six outcomes: success rate of candidemia treatment, candidemia persistence rate, and mortality rates of Candida applicants at 28 and 42 days. None of the six outcomes was influenced by early removal of the CVC (both in 24h and in 48h).211 Based on this study, adult candidemia and the early removal the CVC (24–48h after the start of treatment) cannot be recommended if the patient is receiving an echinocandin and liposomal amphotericin B (B-II). In this case, removal of the CVC is recommended if there is persistent (>72h) isolation of Candida despite treatment.
However, the group consensus considered waiting 72h after the initiation of antifungal therapy to define the need for removal of the CVC, as this cannot be the recommended approach in some scenarios for specific patients. In this sense, in non-neutropenic critically ill patients who have severe sepsis, as well as in breakthrough cases of candidemia in patients receiving more than 3 days of a systemic antifungal agent with activity against the pathogen isolated, early removal of the CVC can be considered (C-III).
Empirical therapyNeutropenic patientsEmpirical antifungal therapy is instituted in neutropenic patients with fever and neutropenia that persist for a period of four to six days after initiation of broad-spectrum antibiotics. This practice was instituted in the 1980s and 1990s, and some randomized trials have been published initially testing this strategy after comparing different agents.212,213 Acceptable options for empirical therapy that have been tested in randomized trials are lipid preparations of amphotericin B, caspofungin and voriconazole.214,215,187 More recently, empirical antifungal therapy has been replaced by another strategy called preemptive therapy, which consists of starting antifungal therapy because of fever and other signs of infection.216 This strategy is more relevant when there is suspicion of infection by filamentous fungi (Aspergillus spp., Fusarium spp. and others). Some biomarkers have been tested, including galactomannan (Aspergillus spp.)217 and 1.3 beta-d-glucan for Candida spp., Aspergillus spp. and other fungi.218
Regarding invasive candidiasis/candidemia, the most important issue to consider in a neutropenic patient with persistent fever despite antibiotic therapy is to assess the risk of infection. There are three parameters to be evaluated: the use of fluconazole in prophylaxis as well as the presence of gastrointestinal mucositis and a CVC. In addition to the risk, another parameter to be considered is the need for coverage of filamentous fungi. Patients with profound neutropenia (>100cells/mm3) lasting more than ten days are those with increased risk for developing filamentous fungal infection.216
Recommendations for empirical therapy for candidemia/invasive candidiasis in neutropenic patientsAmphotericin B deoxycholate should not be used because these patients often have other risk factors for nephrotoxicity, including the underlying disease (e.g., multiple myeloma), its treatment (i.e., anticancer drugs, tumor lysis syndrome) and the use of other nephrotoxic agents (i.e., diuretics, antibiotics) (A-II).183
Patients who are receiving prophylactic fluconazole, do not have gastrointestinal mucositis and who are not at risk of infection by filamentous fungi may not receive empirical antifungal therapy (C-III).219
Patients who are not receiving fluconazole and who are not at risk of infection by filamentous fungus should receive fluconazole (B-I).219 Patients who are receiving fluconazole prophylaxis, yet the clinician considers the possibility of invasive candidiasis, should receive empirical therapy with an agent from another therapeutic class (i.e., a lipid preparation of amphotericin B or an echinocandin – caspofungin or micafungin) (B-II).219
Non-neutropenic patientsCandidemia is an important complication of critically ill patients and is associated with high morbidity and mortality.220,221 Recent studies have shown that the delay in initiating appropriate treatment in patients with candidemia significantly increases mortality.222,223
Approximately 40–50% of candidemias occur in patients admitted to the ICU. This population of patients has a high risk of mortality because they are clinically unstable. Thus, ICU patients at high risk for candidemia/invasive candidiasis may benefit from early initiation of an appropriate antifungal. However, unlike in neutropenic patients, empirical therapy has not been adequately tested in non-neutropenic patients, as there are no validated tools to identify patients at risk and because it is difficult to define outcomes to assess the effectiveness of the therapy.
Despite these limitations, some attempts have been made to identify patients with invasive candidiasis in units of severely ill patients.224–228 These scoring systems use clinical information with or without data from Candida colonization and yielded a reasonable correlation with the occurrence of candidemia/invasive candidiasis. More recently, two biological markers have been tested for the early diagnosis of candidemia/invasive candidiasis: 1-3 beta-d-glucan and PCR. In a study in surgical patients, the evaluation of 1-3 beta-d-glucan in the plasma of patients colonized with Candida was useful to trigger the onset of empirical antifungal.229 In another study, a PCR assay was tested in 225 patients at high risk for candidemia. Using blood culture as the gold standard, the sensitivity and specificity of PCR were 72.1 and 91.2%, respectively.230
Recommendations for empirical therapy for candidemia/invasive candidiasis in non-neutropenic patientsPhysicians should consider the use of empirical antifungal therapy in critically ill patients with risk factors for candidemia and clinical manifestations of infection that are not responding to treatment for bacterial infections (C-III).
The choice of antifungal drug for empirical therapy should be based on the same criteria for the selection of appropriate antifungal treatment for candidemia (see specific section).
To support the clinician in the task of selecting patients for empirical antifungal therapy, as experts, it is our opinion that this therapeutic strategy has a greater chance of success when used in ICU patients with sepsis that is unresponsive to antibiotics (excluding other causes of FOI) who have been exposed to three or more risk factors for candidemia for at least 4–7 days of intensive care, particularly those with Candida colonization in non-sterile sites and a history of major surgery in the last two weeks (C-III).
ProphylaxisNeutropenic and hematopoietic stem cell transplant patientsInvasive candidiasis/candidemia is a frequent complication in neutropenic patients and recipients of HSCTs who do not receive prophylaxis. In neutropenic patients, the frequency varies depending on the patient receiving chemotherapy. The risk factors include neutropenia, the use of a CVC and primarily gastrointestinal mucositis.231 Thus, patients receiving intensive chemotherapy are those with increased risk of developing invasive candidiasis. In HSCT, invasive candidiasis/candidemia typically occurs in two stages: first, early after transplantation, the risk factors are the same as patients receiving chemotherapy, as in this phase, they also have a catheter and neutropenia, and mucositis may develop. After the recovery of the blood marrow, autologous HSCT recipients rarely develop invasive candidiasis/candidemia. The receptors of allogeneic HSCT can present with invasive candidiasis if they develop chronic graft versus host disease (GVHD) in the GI tract.
Several randomized trials testing different agents have been developed for prophylaxis of invasive candidiasis/candidemia in patients receiving both chemotherapy and HSCT. The agents that exhibited efficacy were fluconazole, itraconazole oral solution (but not capsules), voriconazole, posaconazole, micafungin, caspofungin and intravenous amphotericin B. However, many studies have shown no benefits, either due to methodological problems (low numbers of patients) or because the study population had a high risk of developing invasive candidiasis.232
Recommendations for prophylaxis for candidemia/invasive candidiasis in neutropenic patients receiving HSCTHSCTFluconazole is the drug of choice for prophylaxis of invasive candidiasis in the period of neutropenia in recipients of allogeneic HSCT and can be established at the beginning or the end of the conditioning regimen (A-I).233,234 The standard dose is 400mg/day, but there is evidence in a randomized study that 200mg/day is also effective (B-I).235 An alternative to fluconazole is micafungin, but its use is limited by the need for venous access and its high cost (B-I).236
Itraconazole oral solution (not available in Brazil) was also effective, but its use is limited by the high frequency of gastrointestinal side effects (C-I).237,238
Voriconazole is an alternative that can be used when you need coverage for filamentous fungi based on a comparative study with fluconazole (B-I).239
Options for prophylaxis of invasive candidiasis in the post-picks are voriconazole and posaconazole (B-I).240,241
The risk of invasive candidiasis/candidemia is much lower in recipients of autologous HSCT. Thus, prophylaxis is not routinely recommended (C-III). However, prophylaxis (fluconazole) may be indicated in some situations, such as when manipulation of the graft occurs, when severe mucositis is expected, in patients who received fludarabine or cladribine or in those with MBL (mannose-binding lectin) deficiency (B-III).170
NeutropeniaThe results of randomized trials testing fluconazole in neutropenic patients are not as effective as in HSCT, especially because this population is more heterogeneous.232 In general, the more intensive the chemotherapy regimen is, the higher the risk of invasive candidiasis. Thus, patients with acute myeloid leukemia/myelodysplasia receiving remission induction chemotherapy may benefit from prophylaxis. Although fluconazole is the drug of choice for the prevention of invasive candidiasis, these patients also have a high risk of filamentous fungi; thus, posaconazole (200mg orally three times a day) may be preferred (A-I).241
For the prevention of invasive candidiasis, itraconazole oral solution (not available in Brazil) can be used, but it has the limitation of gastrointestinal toxicity (C-I). In a meta-analysis of 13 randomized trials, itraconazole oral solution also prevented the occurrence of invasive aspergillosis, and in ten studies, TCTH receptors were also included.242
Caspofungin was also tested in a randomized study; it is an option, with the exception of requiring venous access for administration (C-I).243
Prophylaxis for invasive candidiasis/candidemia in situations out of remission induction for acute myeloid leukemia/myelodysplasia is not routinely recommended (C-III). However, in special situations, such as after remission induction regimens for acute lymphoid leukemia in high risk patients, prophylaxis may be useful (C-III).
Solid organ transplanted patientsSolid organ transplant recipients represent a set of hosts susceptible to infectious events, which result from the interaction between endogenous immunosuppression (i.e., uremia, diabetes, liver failure), iatrogenic immunosuppression (resulting from the use of medications to prevent rejection episodes) and surgical procedures and their inherent risks. Among infectious events, fungal infections are important because they usually depend on many immunodepression states.
However, the group of transplanted solid organs is heterogeneous with respect to the variables that lead to immunosuppression and, therefore, with respect to the actual state of the resulting immunosuppression, which leads to different rates of fungal infection and different prevalence, including Candida infections.
Epidemiology, clinical significance and recommendations for prophylaxis for candidemia/invasive candidiasis in solid organ transplant patients.
Kidney transplantationRenal transplantation is the most frequent solid organ transplantation and the least technically complex from the surgical point of view because it is an extraperitoneal surgery of short duration.
Renal transplantation is the solid organ transplantation with the lowest rate of invasive Candida infections and the one in which the clinical repercussion is least significant. Approximately 50% of yeast infections are caused by Candida species. Of these, over 70–80% represent urogenital infections (especially candiduria, which occurred in 11% of patients in a retrospective study) or esophagogastric infections. Only 0.5–5% of the infections occur in the form of candidemia or disseminated candidiasis.244
The most prevalent infections (i.e., UTI and esophagitis) are associated with low morbidity and are infections of secondary importance in the spectrum of fungal infections in kidney transplants.
Due to the benign nature of Candida infections in this group and the low rate of candidemia, there is no formal recommendation for chemoprophylaxis.
Exceptions are made for situations in which there is a UTI in the donor at the time of transplantation because there are anecdotal reports of transmission to the donor with serious consequences (i.e., loss of graft anastomosis). Prophylaxis depends on exact timing, and single-agent treatment is not established (C-III).245
Liver transplantationLiver transplantation, the second most frequent solid organ transplantation, is related to high rates of fungal infections (30–40%) mainly due to the complexity of the surgical procedure, which requires an approach through the abdominal cavity and often the bowel, factors known to be related to the occurrence of Candida infections.
Among fungal infections, Candida infections represent 80% of the total events, and candidemia (40%), peritonitis and intracavitary abscesses are the most common manifestations. Most events occur before the sixth post-transplant month, and there has been a reduction in the frequency of Candida over the past years, which has been attributed to improved practices and surgical results.246
Risk factors that distinguish patients at higher risk for invasive candidiasis are retransplantation, dialysis and kidney failure, the need for large volumes of blood products during surgery, antibiotic therapy before transplantation and biliary-enteric anastomosis.246
Contrary to what is observed following kidney transplants, invasive Candida infections are associated with reduced patient survival and considerable morbidity.
In this patient population, randomized, placebo-controlled trials have attempted to reduce invasive candidiasis, reflecting the importance of the event. At least six randomized trials (using fluconazole, itraconazole or liposomal amphotericin) and a meta-analysis of these combined studies are available in the medical literature.247
The results of this meta-analysis, which involved total transplanted groups (with no selection criteria for special groups or subgroups), show total reduction of fungal infections, particularly invasive fungal infections (without specific reference to reducing candidemia), consistent with the results of each individual study and regardless of the antifungal agent used. However, a reduction in mortality is not demonstrated. There is a need for treatment of 11.8 patients to prevent one invasive fungal infection.247
Some authors, having identified heterogeneity in patients and the presence of specific risk factors that identify high-risk populations, advocate focusing on this population as a target for prophylactic therapy.248 However, these recommendations are based on observational and uncontrolled studies, decreasing the strength of the recommendation.
The focus on higher-risk patients is bolstered by the demonstration (from controlled studies) that prophylaxis can lead to side effects, such as the selection of non-albicans strains with greater potential for resistance to azoles.
With the above data available, it is the opinion of this consensus group that antifungal prophylaxis is recommended in liver transplant recipients at greatest risk, recognizing its clinical importance, frequency and the difficulty of establishing the diagnosis in advance. According to the criteria of cost, toxicity and acceptance, we also recommend the use of fluconazole as the drug of choice.249
Find below the specific recommendations.
- •
Patients at risk for whom prophylaxis should be recommended in the first month after transplantation: the existence of at least two of the following risk factors in the first month after transplantation: retransplantation, the need for dialysis, the use of antibiotics and wide biliary-enteric anastomosis (B-II).
- •
Prophylactic scheme: fluconazole 200mg (minimum dose) IV with the possibility of using orally for up to three months, individualized according to the patient's clinical condition (i.e., state of immunosuppression, presence in ICU and persistence of risk factors) (B-II).
- •
Using this strategy, monitor the levels of calcineurin inhibitors (tendency to increase in serum) and check for interactions with other azoles (A-II).
This transplantation modality is also frequently associated with fungal infections because it is performed in diabetic patients and also because of the complexity of the surgery, which involves handling of the intestinal tract.
Over 90% of events are caused by Candida species in the form of intra-abdominal infections with or without concomitant candidemia. As is the case with liver transplantation, invasive Candida infections are associated with both reduced grafts and patient mortality.250
Although the frequency and clinical impact of Candida infections are very similar with respect to what occurs in liver transplantation, there are no randomized studies evaluating the effectiveness of prophylactic antifungal drugs. There are also no studies reporting specific risk factors for the occurrence of fungal infections in this group of transplant recipients. There is only one controlled observational study with historical groups showing lower rates of Candida infections with fluconazole 400mg/day for seven days. The practice is widespread in groups that perform pancreatic transplantation, and there is currently little room for the proposition of controlled studies with placebo.251
It is the opinion of this consensus that prophylaxis should be restricted, recognizing the importance of the event and to curb the excessive use of prophylaxis. Fluconazole can be used in a similar scheme to that used for liver transplantation (C-II).
Thoracic transplantation (heart, lung, heart/lung)In this group of patients, infections occur in 2.2% of patients undergoing heart transplantation and in 9% of patients undergoing lung or heart/lung transplantation. However, unlike what happens with other types of solid organ transplantations, there is a high prevalence of infections by filamentous fungi with high mortality. Candida infections occur in 30% of fungal infections, mainly in the form of hematogenous candidiasis.252
The low incidence of serious fungal infections in heart transplant does not indicate the use of specific prophylaxis in this population.
With respect to lung transplantation, the focus is to prevent the occurrence of filamentous fungi; preventing Candida infection is a less-important goal. Thus, this consensus does not suggest prophylaxis for Candida in this group of patients but reinforces the importance of anti-Aspergillus prophylaxis, which has been adopted by 75% of lung transplantation centers.253
Intestinal transplantationIntestinal transplants are performed infrequently but are associated with high rates of Candida infections by extensive manipulation of the intestinal tract.
Data are scarce regarding prophylaxis in this group; treatment with fluconazole should be considered in high-risk patients.
General recommendationsThere is no indication for routine prophylaxis against Candida in renal transplant patients (B-II). There is evidence for the use of prophylaxis for Candida in liver transplantation with reduction in invasive events but not in mortality (B-II).
Liver transplant patients should receive prophylaxis with fluconazole for one to three months (B-II).
The same level of evidence exists for the use of fluconazole in kidney/pancreas or intestinal transplants, but the use of fluconazole is suggested for high-risk patients (C-III).
There is no indication for routine prophylaxis against Candida in transplanted heart and/or lung patients (B-II).
Non-neutropenic patients in the ICUThere are four randomized and well-designed clinical trials illustrating the benefit of the use of fluconazole in terms of reduction of invasive Candida infection in the ICU, particularly for surgical patients. Despite studies that show the effectiveness of prophylaxis with fluconazole in terms of reduction of invasive Candida infections (but not mortality), it is not possible to establish criteria that are universally applicable for the selection of patients undergoing prophylaxis with this triazole. This fact is due to the large heterogeneity of clinical characteristics in patients admitted to the ICU from different medical centers and the variations in the incidence rates of candidemia in hospitals. Whereas most medical centers have incidence rates of candidemia on the order of 1% among patients in the ICU, 100–200 critically ill patients must be exposed to prophylaxis with fluconazole to prevent one episode of candidemia. In this context, until new criteria for selecting patients at high risk (chance >10% for event) for candidemia are validated, this practice has questionable benefits, as it is associated with increased risk for adverse effects; it also contributes to the development of resistance to triazoles and can lead to increased health care costs.254–256
Conflicts of interestColombo AR developed consultancies for United Medical and MSD laboratories and has participated in Continuing Education activities in MSD, Pfizer, Astellas and United Medical laboratories. Richtmann R lectured in United Medical, MSD and Astellas laboratories. Queiroz-Telles F has participated in Continuing Education activities in Astellas, MSD, Pfizer and United Medical laboratories and in research activities in Astellas, MSD and Pfizer laboratories. Salles MJ has taught classes and courses of Continuing Education in Pfizer, MSD, Gilead, Sanofi-Aventis and Novartis laboratories. Cunha CA is a member of the advisory board in MSD, United Medical and Schering-Plough laboratories, lectured in Pfizer, MSD, United Medical, Bago, Schering-Plough and Astellas laboratories and developed clinical research in Pfizer, Schering-Plough, Astellas and Basilea Pharmaceutica laboratories. Nucci M developed consultancies and lectured in MSD, Pfizer, Astellas and United Medical laboratories and has received honoraria for research in MSD and Pfizer laboratories. Guimarães T, Camargo LF, Yasuda MA, and Moretti ML declare to have no conflicts of interest.
Coordinators: Arnaldo Lopes Colombo (Universidade Federal de São Paulo), Luis Fernando Aranha Camargo (Universidade Federal de São Paulo), Thaís Guimarães (Hospital do Servidor Público Estadual de São Paulo/HC-FMUSP).
Participants: Anna Sara Levin (HC-FMUSP), Arnaldo Lopes Colombo (Universidade Federal de São Paulo), Claudia Maffei (Universidade de São Paulo – Campus Ribeirão Preto), Claudia Mangini (Hospital Vivalle), Clóvis Arns da Cunha (Universidade Federal do Paraná), Flavio de Queiroz-Telles (Hospital de Clínicas, Universidade Federal do Paraná), Luis Fernando Aranha Camargo (Universidade Federal de São Paulo), Marcelo Magri (HC-FMUSP), Marcio Nucci (Universidade Federal do Rio de Janeiro), Maria Aparecida Shikanai Yasuda (Universidade de São Paulo), Maria Luiza Moretti (Universidade Estadual de Campinas), Mauro José Costa Salles (Santa Casa de Misericórdia de São Paulo), Rosana Richtmann (Instituto de Infectologia Emilio Ribas/Pro-Matre-Santa Joana), Roseli Calil (Universidade Estadual de Campinas), Thaís Guimarães (Hospital do Servidor Público Estadual de São Paulo/HC-FMUSP), Zarifa Khouri (Instituto de Infectologia Emilio Ribas).