Neisseria meningitidis is a bacterium that colonizes the human nasopharynx and is transmitted by respiratory droplets from asymptomatic or symptomatic carriers. Occasionally, the pathogen invades the mucosa and enters the bloodstream, causing invasive meningococcal disease, a life-threatening infection. While meningococcal colonization is the first step in the development of invasive disease, the risk factors that predict progression from asymptomatic to symptomatic status are not well-known. The present report aimed to describe the prevalence of N. meningitidis carriers throughout the Americas, emphasizing the risk factors associated with carrier status, as well as the most prevalent serogroups in each studied population. We conducted a systematic review by searching for original studies in the MEDLINE/PubMed, Embase, LILACS and SciELO databases, published between 2001 and 2018. Exclusion criteria were articles published in a review format, case studies, case control studies, investigations involving animal models, and techniques or publications that did not address the prevalence of asymptomatic carriers in an American country. A total of 784 articles were identified, of which 23 were selected. The results indicate that the highest prevalence rates are concentrated in Cuba (31.9%), the United States (24%), and Brazil (21.5%), with increased prevalence found among adolescents and young adults, specifically university students and males. The present systematic review was designed to support epidemiological surveillance and prevention measures to aid in the formulation of strategies designed to control the transmission of meningococci in a variety of populations and countries throughout the Americas.
Neisseria meningitidis or meningococcus is a diplococcus Gram-negative bacterium that is known to colonize the human nasopharynx of approximately 10% of the population at any given time.1 Transmission occurs via the inhalation of respiratory droplets or through direct contact with nasopharynx secretions from asymptomatic or symptomatic carriers.2 Occasionally, the pathogen invades the mucosa and enters the bloodstream to cause invasive meningococcal disease (IMD), such as meningitis or septicemia.3,4 Less common manifestations of meningococcal disease include myocarditis, endocarditis and pericarditis.4
N. meningitidis strains are categorized into 12 capsular types (A, B, C, E, H, I, K, L, W, X, Y and Z), according to the biochemical composition and structure of capsular polysaccharide.5 Six serogroups (A, B, C, W, X, and Y) are responsible for the majority of IMD cases, and serogroup prevalence varies temporarily and by geographic location.5,6
Meningococcal colonization is the initial step in the development of IMD.3 As a consequence, since the middle of the 20th century, studies have been carried out worldwide to analyze the prevalence of asymptomatic carriers of N. meningitidis in order to better understand the transmission process, the epidemiology of this disease and to obtain information to improve vaccination strategies. On the other hand, differences in carrier profiles throughout the American continent, both in terms of prevalence and the population(s) affected, can interfere with the correlation of existing data.
Currently, three types of meningococcal vaccine exist: polysaccharide vaccine, conjugate vaccine, and multi-peptide vaccine.7,8 The polysaccharide vaccine, which is composed of capsule antigens, is falling into disuse due to low immunogenicity.9 The conjugate vaccine, developed by the conjugation of polysaccharide antigens with carrier proteins, may exert an effect known as herd protection, which promotes defense against transmission.10 The multi-peptide vaccine, produced from outer membrane vesicles and subcapsular proteins, was developed to respond to epidemics mainly arising from serogroup B.11
Investigations into meningococcal carriage are crucial to furthering the understanding of transmission dynamics and epidemiology, as well as the potential effects of control programs, such as vaccination.12 At the time of this manuscript elaboration, no article reviews focusing on meningococcal carriage studies in the Americas were found in the literature. The compilation of these studies could provide insight into the epidemiology of meningococcal disease throughout this region, and could assist in the development of targeted strategies to reduce the transmission of specific meningococcal strains. Therefore, the aim of this systematic review was to provide information regarding the prevalence of N. meningitidis carriers in the Americas, emphasizing the risk factors associated with carrier status and the most prevalent serogroups in each studied population.
MethodsA systematic review was conducted to identify the prevalence of meningococcal carriage in studies performed in countries located throughout the Americas. The proposed question was: What is the prevalence and associated risk factors for N. meningitidis carriage among people living in American countries? This study was carried out in accordance with the recommendations established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyzes—PRISMA,13 and was also registered in the PROSPERO database under number CRD42018106755.
Search strategyTwo authors (JFSN and MSMS) consulted the following databases: Embase, MEDLINE/PubMed and LILACS. SciELO was used as a supplementary source.
The search strategy employed a combination of descriptors and keywords adapted to each database. The main keywords used were N. meningitidis, Carrier and Carriage. The detailed search strategy is described in Table 1. MeSH terms were used to improve searches in PubMed, while Health Science Descriptors (DeCS) were used to search the SciELO and LILACS databases.
Detailed search strategy.
| Database | Search strategy |
|---|---|
| MEDLINE/PubMed | ("neisseria meningitidis"[MeSH Terms] OR "neisseria meningitidis"[TIAB]) AND (Carrie* OR Carriage OR "Carrier State"[Mesh] OR "epidemiology"[Subheading] OR epidemiology[TIAB] OR "epidemiology"[MeSH Terms]) |
| Date run: September 24th, 2018 | AND |
| October 28th, 2018 | ("neisseria meningitidis"[MeSH Terms] OR "neisseria meningANGUILLA[TIAB] OR ANGUILLA[AD] OR "Antigua and Barbuda"[MESH] OR ANTIGUA[TIAB] OR ANTIGUA[AD] OR "ARGENTINA"[MESH] OR ARGENTINA[TIAB] OR ARGENTINA[AD] OR ARGENTINE[TIAB] OR ARGENTINO[TIAB] OR ARUBA[TIAB] OR ARUBA[AD] OR "bahamas"[MeSH Terms] OR "bahamas"[TIAB] OR BAHAMAS[AD] OR "barbados"[MeSH Terms] OR "barbados"[TIAB] OR BARBADOS[AD] OR "belize"[MeSH Terms] OR "belize"[TIAB] OR BELIZE[AD] OR belize OR bonnaire OR "San Eustaquio" OR eustatius OR "chile"[MeSH Terms] OR "chile"[TIAB] OR CHILE[AD] OR "Costa Rica"[MeSH Terms] OR "costa rica"[TIAB] OR "COSTA RICA"[AD] OR "CUBA"[MESH] OR CUBA[TIAB] OR CUBA[AD] OR curacao OR "DOMINICA"[MESH] OR DOMINICA[TIAB] OR DOMINICA[AD] OR "grenada"[MeSH Terms] OR "grenada"[TIAB] OR GRENADA[AD] OR granada OR guadalupe OR "GUADELOUPE"[MESH] OR GUADELOUPE[TIAB] OR GUADELOUPE[AD] OR "Turks and Caicos Islands" OR "Virgin Islands of the United States"[All Fields] OR "united states virgin islands"[MESH] OR "Virgin Islands"[TIAB] OR "Virgin Islands"[AD] OR "jamaica"[MeSH Terms] OR "jamaica"[TIAB] OR JAMAICA[AD] OR "MARTINIQUE"[MESH] OR MARTINIQUE[TIAB] OR MARTINIQUE[AD] OR "PUERTO RICO"[MESH] OR "PUERTO RICO"[TIAB] OR "PUERTO RICO"[AD] OR "Saint Kitts and Nevis"[TIAB] OR "St. Kitts"[TIAB] OR "St. Kitts"[AD] OR "saint kitts and nevis"[MeSH Terms] OR "Saint Lucia"[MESH] OR "Saint Lucia"[TIAB] OR "Saint Lucia"[AD] OR "SANTA LUCIA" OR "Saint Vincent and the Grenadines"[MESH] OR "Saint Vincent and the Grenadines"[TIAB] OR "Saint Vincent and the Grenadines"[AD] OR "Saint Martin"[TIAB] OR "Saint Martin"[AD]OR "Sint Maarten"[AD] OR "Saint-Martin"[AD] OR "Saint-Martin"[TIAB] OR "suriname"[MeSH Terms] OR "surinam*"[TIAB] OR SURINAM*[AD] OR "Trinidad and Tobago"[MESH] OR "Trinidad and Tobago"[TIAB] OR "Trinidad and Tobago"[AD] OR "TRINIDAD TOBAGO" OR "uruguay"[MeSH Terms] OR "uruguay"[TIAB] URUGUAY[AD] OR "haiti"[MeSH Terms] OR "haiti"[TIAB] OR HAITI[AD] OR "brazil"[MeSH Terms] OR BRAZIL*[TIAB] OR BRAZIL[AD] OR BRASIL[TIAB] OR BRASIL[AD] OR "colombia"[MeSH Terms] OR colombia*[TIAB] OR COLOMBIA[AD] OR dominican*[TIAB] OR DOMINICAN*[AD] OR "Dominican Republic"[TIAB] OR "Dominican Republic"[AD] OR "El Salvador"[TIAB] OR "EL SALVADOR"[AD] OR "guyana"[MeSH Terms] OR "guyana"[TIAB] OR GUYANA[AD] OR GUIANA[TIAB] OR GUIANA[AD] OR "honduras"[MeSH Terms] OR "honduras"[TIAB] OR HONDURAS[AD] OR HONDURANS[TIAB] O |
| OR | |
| HONDURANS[TIAB] OR "mexico"[MeSH Terms] OR mexic*[TIAB] OR MEXICO[AD] OR "panama"[MeSH Terms] OR "panama"[TIAB] OR PANAMA[AD] OR "paraguay"[MeSH Terms] OR paraguay*[TIAB] OR PARAGUAY[AD] OR "venezuela"[MeSH Terms] OR Venezuela*[TIAB] OR VENEZUELA[AD] OR "bolivia"[MeSH Terms] OR BOLIVIA*[TIAB] OR BOLIVIA[AD] OR "ecuador"[MeSH Terms] OR "ecuador"[TIAB] OR ECUADOR[AD] OR EQUATOR[AD] OR EQUATORIAN[TIAB] OR "guatemala"[MeSH Terms] OR "guatemala"[AD] OR GUATEMAL*[TIAB] OR "nicaragua"[MeSH Terms] OR "nicaragua"[AD] OR NICARAGUA*[TIAB] OR "peru"[MeSH Terms] OR "peru"[AD] OR PERU[TIAB] OR PERUVIAN[TIAB] OR ("cayman"[All Fields] AND "islands"[All Fields]) OR "cayman islands"[All Fields] OR Canada OR CANADIAN OR UNITED STATES OR AMERICAN OR USA[TIAB] OR NORTH AMERICA) | |
| EMBASE | (Carrie* OR Carriage) AND Neisseria meningitidis AND (Prevalence OR Epidemiology) |
| Date run: September 20th, 2018 | Geographical limit strategy from PubMed was adapted and used in this database. |
| Publication dates: 2001–2018. | |
| Type of document: article + article in press | |
| LILACS | (Portador or carrie$ OR carriage or prevalenc$) AND Neisseria meningitidis |
| Date run: September 24th, 2018 | Geographical limit not used because this is a regional database. |
| October 10th, 2018 | Publication year: 2001–2017 |
| Type of document: article | |
| SciELO | (Portador OR carrie* OR carriage OR prevalenc*) AND Neisseria meningitidis |
| Date run: September 16th, 2018 | Publication year: 2001–2018 |
| October 10th, 2018 | Literature type: article |
Studies were included in the descriptive synthesis if published as original articles between January 1, 2001 and September 24, 2018. This period was chosen due to a higher number of carrier studies conducted in the 21st century in the Americas assessing risk factors related to social behaviors of the populations studied. The language the study was written in was not considered as a criterion for inclusion.
Exclusion criteriaExclusion criteria consisted of articles in review format, case reports, case-control studies, articles employing animal models, case studies or publications focusing on isolation techniques or individual strains, or publications that did not address the prevalence of asymptomatic carriers in American countries.
Study selection and data collectionThe identified studies were imported to EndNote X8, where records were organized, and duplicates were excluded. Data extraction included information on first author, year of specimen collection, the country in which the study was conducted, age, study design, swab site, reported prevalence, vaccination status, and serogroup identification.
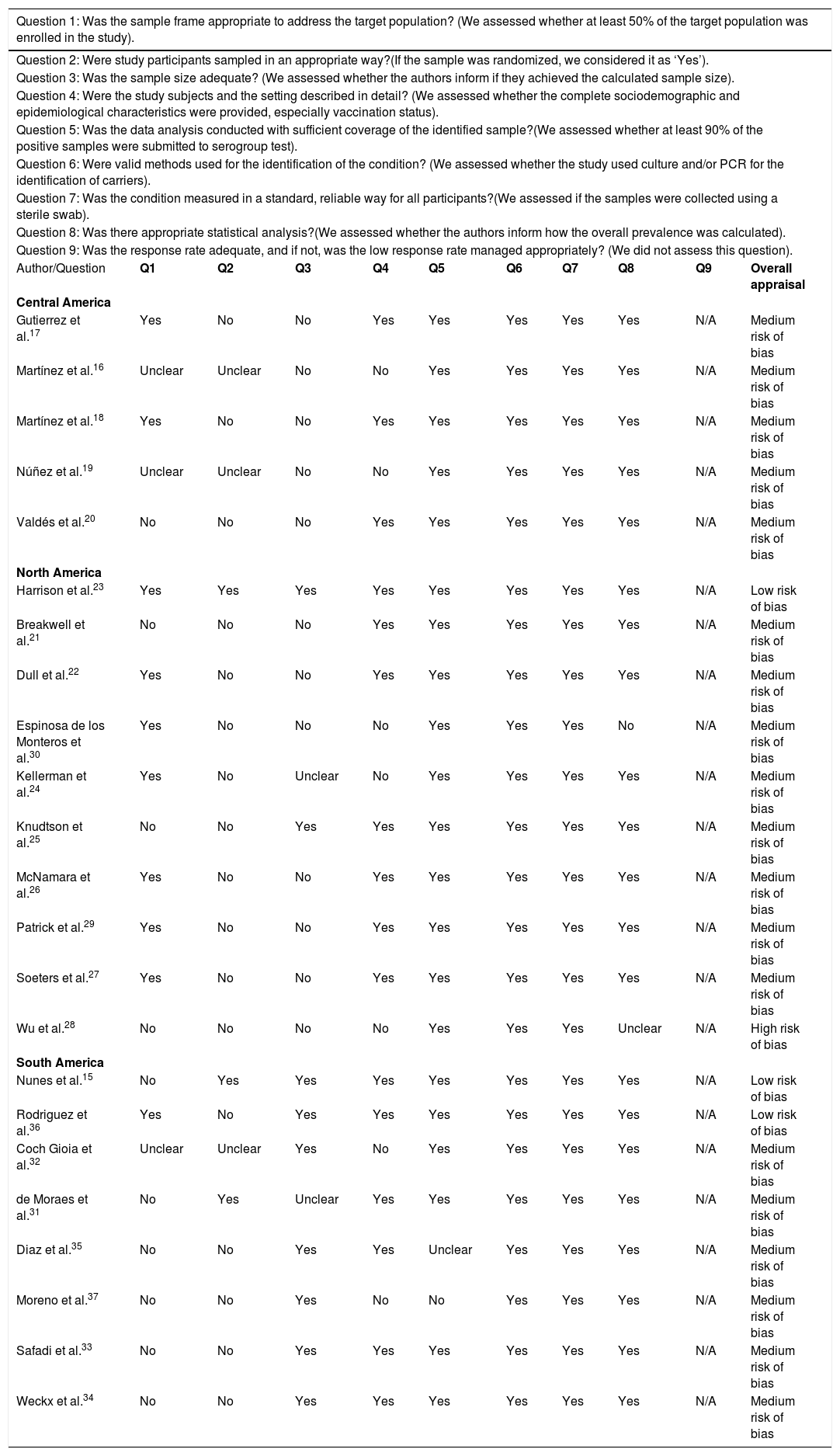
Quality assessmentStudy quality was assessed using the Joanna Briggs Institute Critical Appraisal Checklist for Studies Reporting Prevalence Data,14 which includes nine questions regarding study objective, methodology and results. The final question on the checklist was excluded, since response rate was not a factor consistently evaluated in the articles included in this review, as convenience sampling was employed in the vast majority of studies considered herein. To evaluate the methodological quality of the articles using a quality scoring system, studies were ranked in terms of bias, with 0–3 questions answered “yes” indicating a high risk of bias, 4–6 as medium risk, and 7–8 as presenting a low risk of bias.
Data analysisThe present review attempted to provide a descriptive synthesis of the findings reported by the included studies, focusing on the prevalence of carriers and characteristics of the populations evaluated in each country. In an effort to achieve more specific findings, we recalculated the reported prevalence ratios when necessary. The vaccine coverage data obtained from the articles was also recalculated to identify the percentage of vaccinated carriers by dividing the number of vaccinated carriers by the total number of carriers.
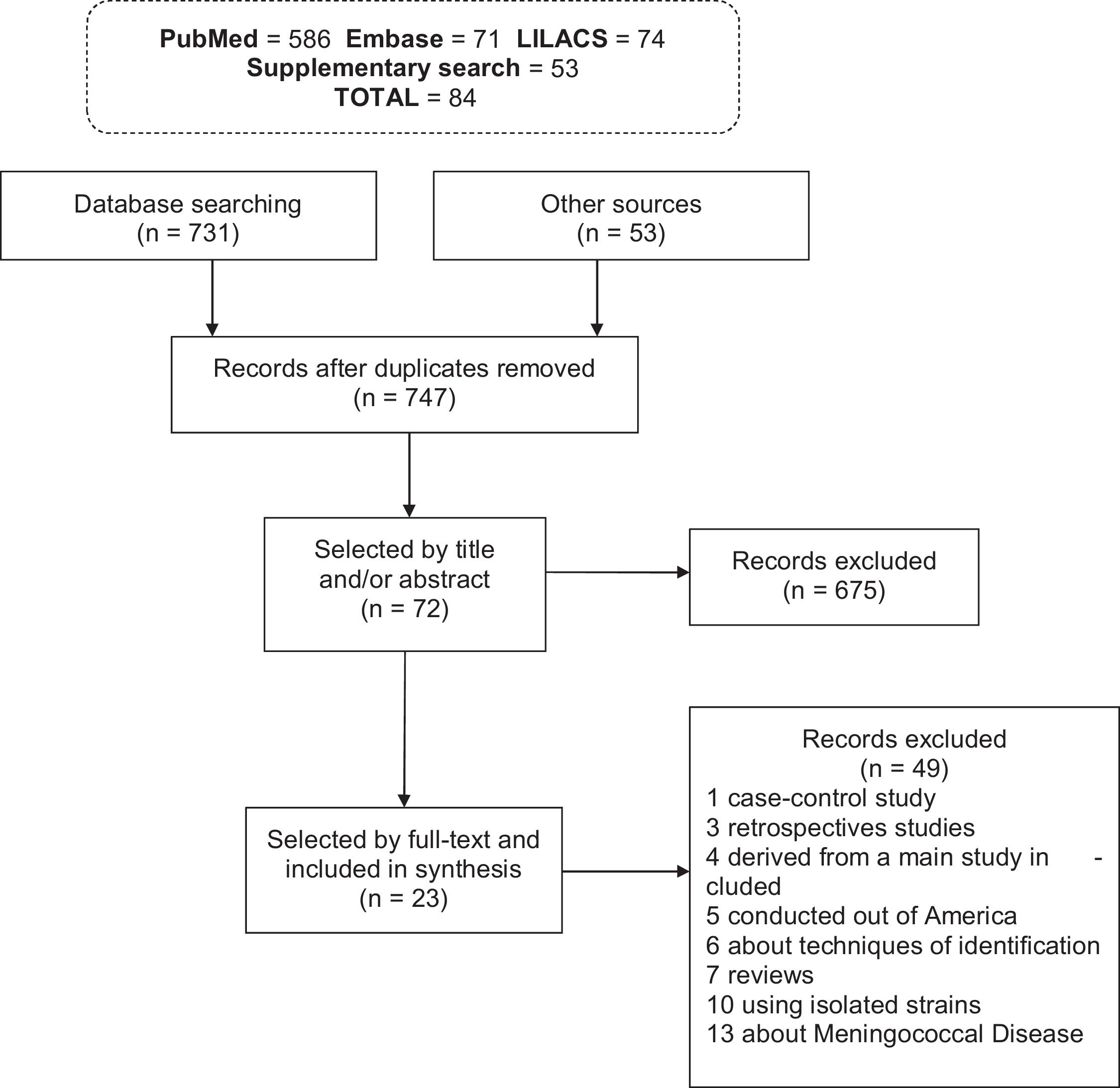
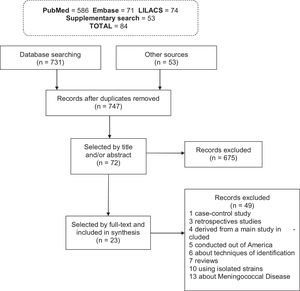
ResultsStudies characteristicsThe search strategy initially identified 784 records: 731 records from databases and 53 from additional sources. Duplicate records were evaluated, resulting in 37 articles being excluded. From the remaining 747 records, 724 articles were excluded due to non-conformance with the inclusion criteria (Fig. 1). Of the 23 studies included in total, 18 (78.3%) were identified in MEDLINE/PubMed, 3 (13%) in SciELO, 2 (8.7%) in LILACS, while no articles were included from the Embase database.
Among the 23 articles analyzed, six were conducted in Central America, 10 in North America and eight in South America. Most studies (43.5%; n = 10/23) evaluated swabs collected from the oropharynx and just seven (30.4%) provided no information about vaccination.
Almost all the serogroup identification included were evaluated by slide agglutination, except one that was performed using only polymerase chain reaction and whole-genome sequencing to identify the genogroups.15
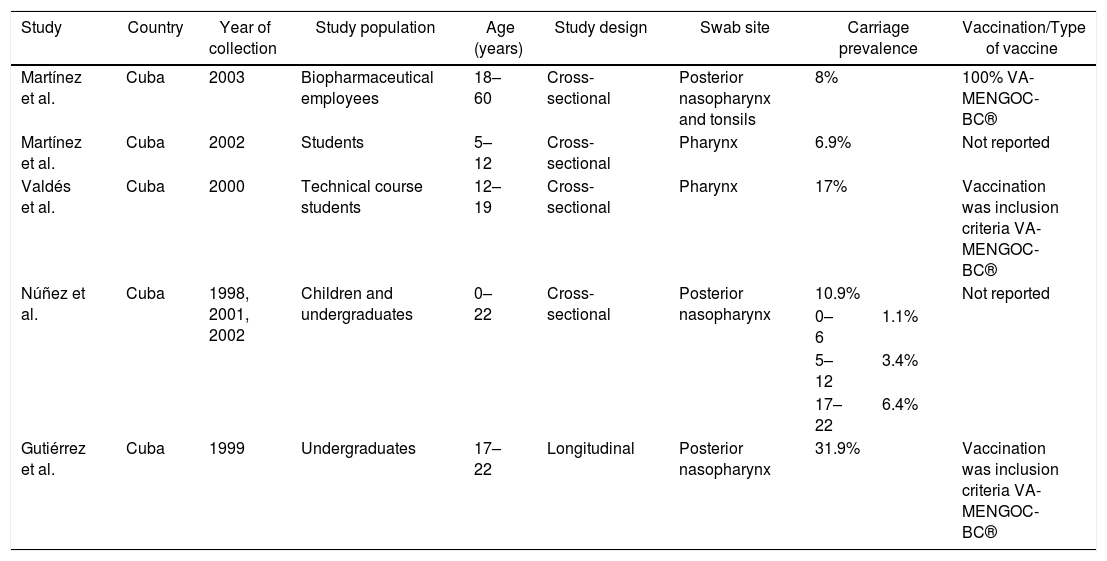
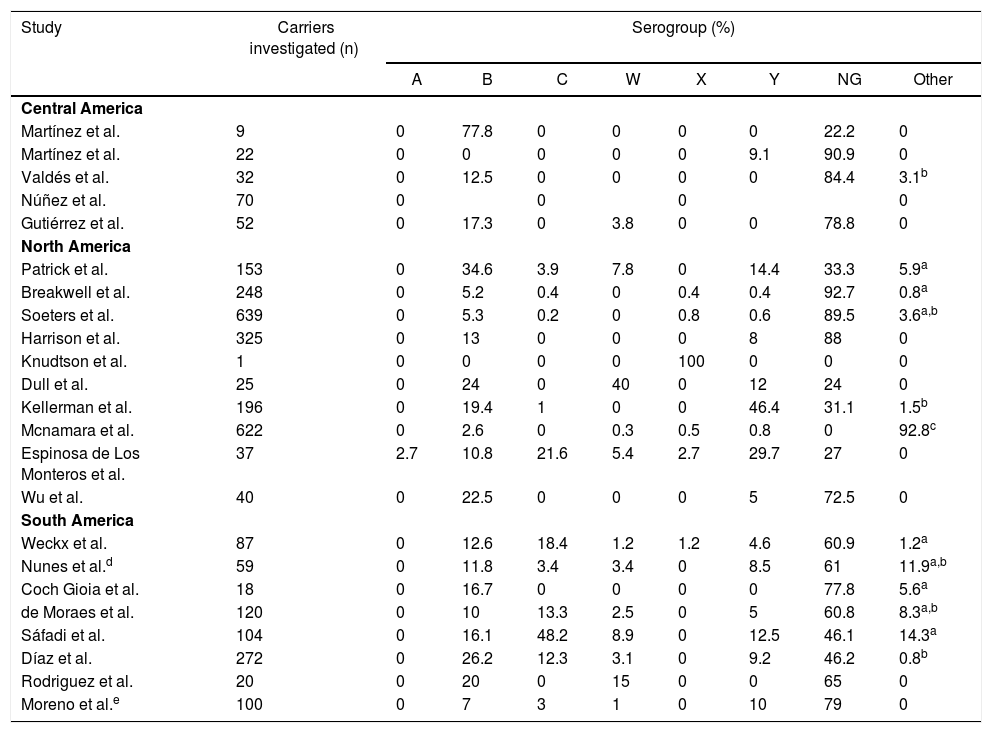
Carriage according to subcontinentCentral AmericaN. meningitidis carriage studies were only identified in Cuba (Table 2).16–20 No recent data was available, as the latest study was performed in 2003.16 The reported prevalence was not similar among the studies, ranging from 6.9%18 to 31.9%.17 Most N. meningitidis isolates were identified as non-groupable or as serogroup B (Table 3). Vaccination was used as inclusion criteria in two of the studies17,20 and another one reported high coverage.19 In all of these studies, the vaccine reported was VA-MENGOC-BC®. Two studies showed an association between meningococcal carriage and male sex and age,18,19 while another showed an association with recent influenza-like illness.15
Characteristics from studies conducted in Central America.
| Study | Country | Year of collection | Study population | Age (years) | Study design | Swab site | Carriage prevalence | Vaccination/Type of vaccine | |
|---|---|---|---|---|---|---|---|---|---|
| Martínez et al. | Cuba | 2003 | Biopharmaceutical employees | 18–60 | Cross-sectional | Posterior nasopharynx and tonsils | 8% | 100% VA-MENGOC-BC® | |
| Martínez et al. | Cuba | 2002 | Students | 5–12 | Cross-sectional | Pharynx | 6.9% | Not reported | |
| Valdés et al. | Cuba | 2000 | Technical course students | 12–19 | Cross-sectional | Pharynx | 17% | Vaccination was inclusion criteria VA-MENGOC-BC® | |
| Núñez et al. | Cuba | 1998, 2001, 2002 | Children and undergraduates | 0–22 | Cross-sectional | Posterior nasopharynx | 10.9% | Not reported | |
| 0–6 | 1.1% | ||||||||
| 5–12 | 3.4% | ||||||||
| 17–22 | 6.4% | ||||||||
| Gutiérrez et al. | Cuba | 1999 | Undergraduates | 17–22 | Longitudinal | Posterior nasopharynx | 31.9% | Vaccination was inclusion criteria VA-MENGOC-BC® | |
Serogroup prevalence by region.
| Study | Carriers investigated (n) | Serogroup (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | W | X | Y | NG | Other | ||
| Central America | |||||||||
| Martínez et al. | 9 | 0 | 77.8 | 0 | 0 | 0 | 0 | 22.2 | 0 |
| Martínez et al. | 22 | 0 | 0 | 0 | 0 | 0 | 9.1 | 90.9 | 0 |
| Valdés et al. | 32 | 0 | 12.5 | 0 | 0 | 0 | 0 | 84.4 | 3.1b |
| Núñez et al. | 70 | 0 | 0 | 0 | 0 | ||||
| Gutiérrez et al. | 52 | 0 | 17.3 | 0 | 3.8 | 0 | 0 | 78.8 | 0 |
| North America | |||||||||
| Patrick et al. | 153 | 0 | 34.6 | 3.9 | 7.8 | 0 | 14.4 | 33.3 | 5.9a |
| Breakwell et al. | 248 | 0 | 5.2 | 0.4 | 0 | 0.4 | 0.4 | 92.7 | 0.8a |
| Soeters et al. | 639 | 0 | 5.3 | 0.2 | 0 | 0.8 | 0.6 | 89.5 | 3.6a,b |
| Harrison et al. | 325 | 0 | 13 | 0 | 0 | 0 | 8 | 88 | 0 |
| Knudtson et al. | 1 | 0 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| Dull et al. | 25 | 0 | 24 | 0 | 40 | 0 | 12 | 24 | 0 |
| Kellerman et al. | 196 | 0 | 19.4 | 1 | 0 | 0 | 46.4 | 31.1 | 1.5b |
| Mcnamara et al. | 622 | 0 | 2.6 | 0 | 0.3 | 0.5 | 0.8 | 0 | 92.8c |
| Espinosa de Los Monteros et al. | 37 | 2.7 | 10.8 | 21.6 | 5.4 | 2.7 | 29.7 | 27 | 0 |
| Wu et al. | 40 | 0 | 22.5 | 0 | 0 | 0 | 5 | 72.5 | 0 |
| South America | |||||||||
| Weckx et al. | 87 | 0 | 12.6 | 18.4 | 1.2 | 1.2 | 4.6 | 60.9 | 1.2a |
| Nunes et al.d | 59 | 0 | 11.8 | 3.4 | 3.4 | 0 | 8.5 | 61 | 11.9a,b |
| Coch Gioia et al. | 18 | 0 | 16.7 | 0 | 0 | 0 | 0 | 77.8 | 5.6a |
| de Moraes et al. | 120 | 0 | 10 | 13.3 | 2.5 | 0 | 5 | 60.8 | 8.3a,b |
| Sáfadi et al. | 104 | 0 | 16.1 | 48.2 | 8.9 | 0 | 12.5 | 46.1 | 14.3a |
| Díaz et al. | 272 | 0 | 26.2 | 12.3 | 3.1 | 0 | 9.2 | 46.2 | 0.8b |
| Rodriguez et al. | 20 | 0 | 20 | 0 | 15 | 0 | 0 | 65 | 0 |
| Moreno et al.e | 100 | 0 | 7 | 3 | 1 | 0 | 10 | 79 | 0 |
NG: Non-groupable; Other: serogroups E, H, I, K, L and Z; Blank spaces: the article does not inform the value.
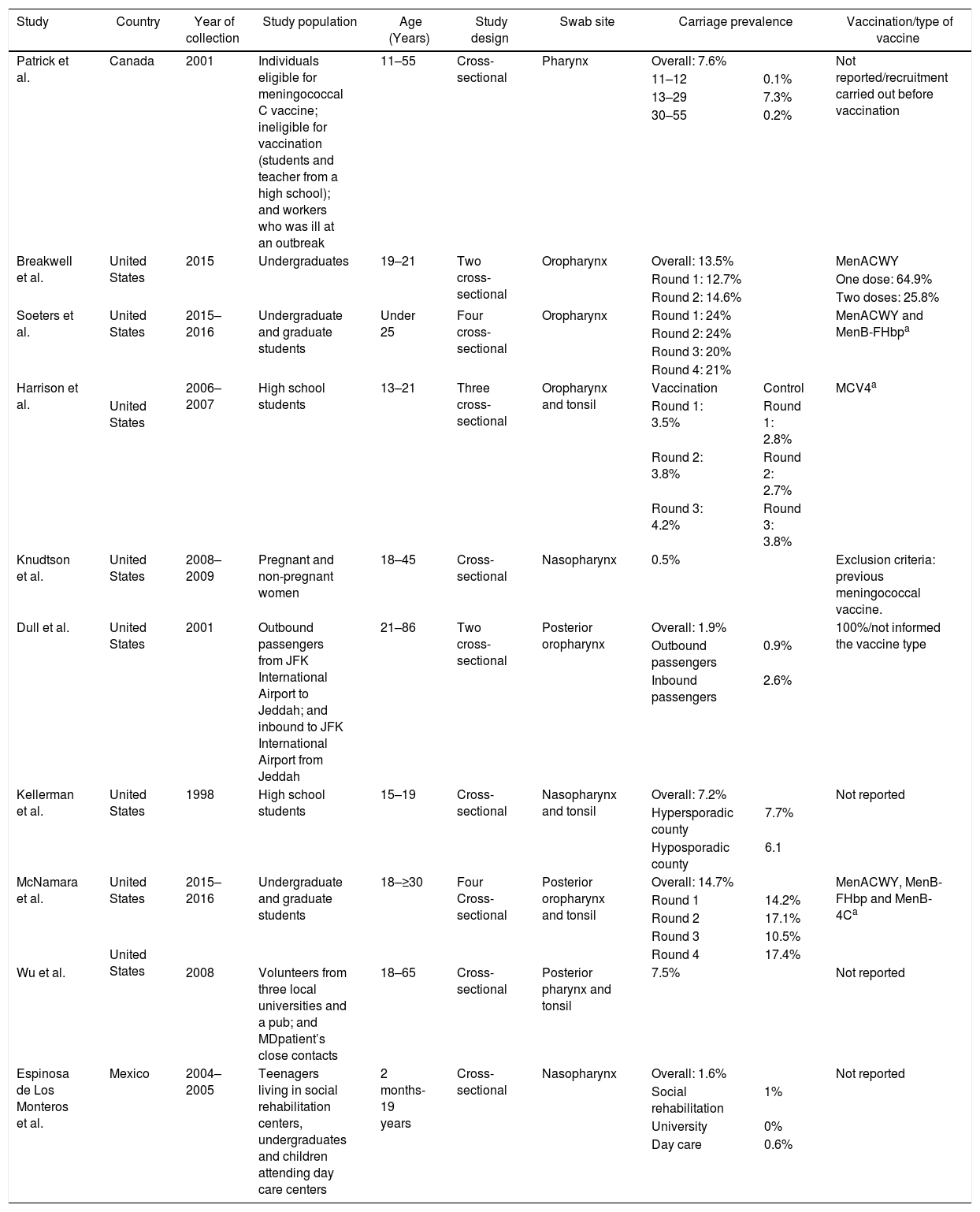
Several studies were conducted in North America, mostly in the United States,21–28 but also in Canada29 and Mexico (Table 4).30 The lowest overall carriage prevalence was found in a study comparing pregnant and non-pregnant women (0.5%)25; in addition, the highest prevalence was found among undergraduate and graduate students (24%).27 Most of the N. meningitidis isolates were found to be non-groupable, although other serogroups not normally associated with meningococcal disease have also been reported, such as the serogroup E reported in Canadian study (Table 3).29 Just three studies did not report vaccination status24,28,30 and one employed previous vaccination as exclusion criteria.25 Those that reported vaccination indicated high vaccine coverage with the quadrivalent conjugate vaccine (MenACWY). Meningococcal carriage was reported in association with male sex,21,24,26,27,29 age,23,29,30 antibiotic usage,21,26,27 current exposure to cigarette smoke,21,23,25,26,27 level of parental education,23 household agglomeration condition,25 upper respiratory infection,21 and attendance at pubs/parties.21,26,27
Characteristics from studies conducted in North America.
| Study | Country | Year of collection | Study population | Age (Years) | Study design | Swab site | Carriage prevalence | Vaccination/type of vaccine | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patrick et al. | Canada | 2001 | Individuals eligible for meningococcal C vaccine; ineligible for vaccination (students and teacher from a high school); and workers who was ill at an outbreak | 11–55 | Cross-sectional | Pharynx | Overall: 7.6% | Not reported/recruitment carried out before vaccination | |||
| 11–12 | 0.1% | ||||||||||
| 13–29 | 7.3% | ||||||||||
| 30–55 | 0.2% | ||||||||||
| Breakwell et al. | United States | 2015 | Undergraduates | 19–21 | Two cross-sectional | Oropharynx | Overall: 13.5% | MenACWY | |||
| Round 1: 12.7% | One dose: 64.9% | ||||||||||
| Round 2: 14.6% | Two doses: 25.8% | ||||||||||
| Soeters et al. | United States | 2015–2016 | Undergraduate and graduate students | Under 25 | Four cross-sectional | Oropharynx | Round 1: 24% | MenACWY and MenB-FHbpa | |||
| Round 2: 24% | |||||||||||
| Round 3: 20% | |||||||||||
| Round 4: 21% | |||||||||||
| Harrison et al. | 2006–2007 | High school students | 13–21 | Three cross-sectional | Oropharynx and tonsil | Vaccination | Control | MCV4a | |||
| United States | Round 1: 3.5% | Round 1: 2.8% | |||||||||
| Round 2: 3.8% | Round 2: 2.7% | ||||||||||
| Round 3: 4.2% | Round 3: 3.8% | ||||||||||
| Knudtson et al. | United States | 2008–2009 | Pregnant and non-pregnant women | 18–45 | Cross-sectional | Nasopharynx | 0.5% | Exclusion criteria: previous meningococcal vaccine. | |||
| Dull et al. | United States | 2001 | Outbound passengers from JFK International Airport to Jeddah; and inbound to JFK International Airport from Jeddah | 21–86 | Two cross-sectional | Posterior oropharynx | Overall: 1.9% | 100%/not informed the vaccine type | |||
| Outbound passengers | 0.9% | ||||||||||
| Inbound passengers | 2.6% | ||||||||||
| Kellerman et al. | United States | 1998 | High school students | 15–19 | Cross-sectional | Nasopharynx and tonsil | Overall: 7.2% | Not reported | |||
| Hypersporadic county | 7.7% | ||||||||||
| Hyposporadic county | 6.1 | ||||||||||
| McNamara et al. | United States | 2015–2016 | Undergraduate and graduate students | 18–≥30 | Four Cross-sectional | Posterior oropharynx and tonsil | Overall: 14.7% | MenACWY, MenB-FHbp and MenB-4Ca | |||
| Round 1 | 14.2% | ||||||||||
| Round 2 | 17.1% | ||||||||||
| Round 3 | 10.5% | ||||||||||
| United States | Round 4 | 17.4% | |||||||||
| Wu et al. | 2008 | Volunteers from three local universities and a pub; and MDpatient’s close contacts | 18–65 | Cross-sectional | Posterior pharynx and tonsil | 7.5% | Not reported | ||||
| Espinosa de Los Monteros et al. | Mexico | 2004–2005 | Teenagers living in social rehabilitation centers, undergraduates and children attending day care centers | 2 months-19 years | Cross-sectional | Nasopharynx | Overall: 1.6% | Not reported | |||
| Social rehabilitation | 1% | ||||||||||
| University | 0% | ||||||||||
| Day care | 0.6% | ||||||||||
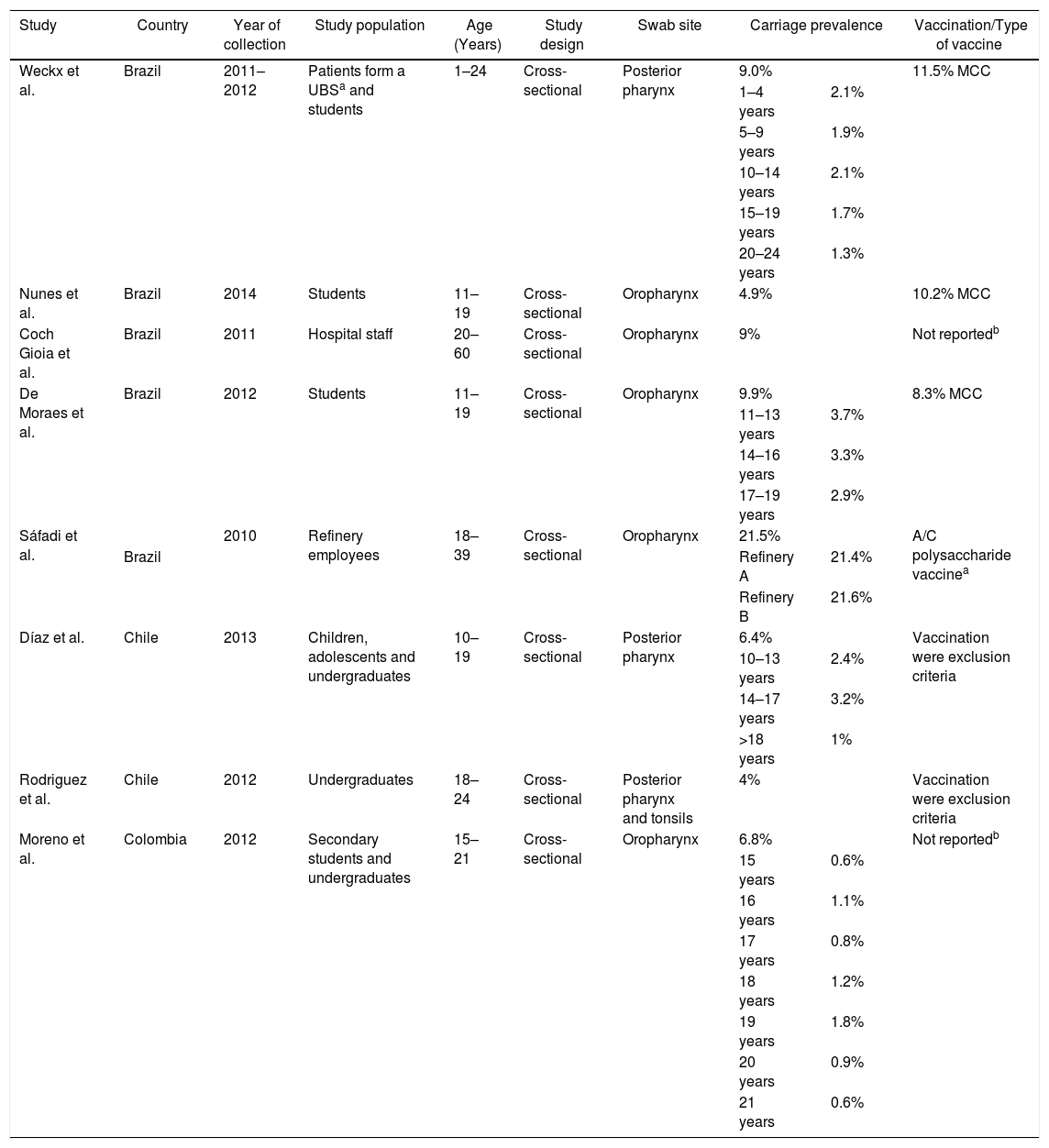
Meningococcal carriage was assessed in Brazil,15,31–34 Chile35,36 and Colombia37 among different populations (Table 5). The highest rate found was 21.5% among refinery employees in Brazil,33 while the lowest was 4% among undergraduates in Chile.36 Non-groupable strains of N. meningitidis were most commonly described, followed by serogroups B and C (Table 3). Vaccination status was identified in four studies, with three reporting relatively low MCC coverage,15,31–34 while one showed high coverage of the meningococcal A/C polysaccharide vaccine.33 Two studies employed previous vaccination as exclusion criteria35,36 and two did not report any information regarding vaccination coverage in the studied populations.32,37 Only one study demonstrated an association between meningococcal carriage and male sex, whereas other risk factors were reported in other studies, such as current exposure to cigarette smoke,15,31,35,37 level of parental education,31,33 household agglomeration condition,15,34,35,36 upper respiratory infection,31 oral sex,37 and attendance at pubs/parties.15,31,32,36
Characteristics from studies conducted in South America.
| Study | Country | Year of collection | Study population | Age (Years) | Study design | Swab site | Carriage prevalence | Vaccination/Type of vaccine | |
|---|---|---|---|---|---|---|---|---|---|
| Weckx et al. | Brazil | 2011–2012 | Patients form a UBSa and students | 1–24 | Cross-sectional | Posterior pharynx | 9.0% | 11.5% MCC | |
| 1–4 years | 2.1% | ||||||||
| 5–9 years | 1.9% | ||||||||
| 10–14 years | 2.1% | ||||||||
| 15–19 years | 1.7% | ||||||||
| 20–24 years | 1.3% | ||||||||
| Nunes et al. | Brazil | 2014 | Students | 11–19 | Cross-sectional | Oropharynx | 4.9% | 10.2% MCC | |
| Coch Gioia et al. | Brazil | 2011 | Hospital staff | 20–60 | Cross-sectional | Oropharynx | 9% | Not reportedb | |
| De Moraes et al. | Brazil | 2012 | Students | 11–19 | Cross-sectional | Oropharynx | 9.9% | 8.3% MCC | |
| 11–13 years | 3.7% | ||||||||
| 14–16 years | 3.3% | ||||||||
| 17–19 years | 2.9% | ||||||||
| Sáfadi et al. | 2010 | Refinery employees | 18–39 | Cross-sectional | Oropharynx | 21.5% | A/C polysaccharide vaccinea | ||
| Brazil | Refinery A | 21.4% | |||||||
| Refinery B | 21.6% | ||||||||
| Díaz et al. | Chile | 2013 | Children, adolescents and undergraduates | 10–19 | Cross-sectional | Posterior pharynx | 6.4% | Vaccination were exclusion criteria | |
| 10–13 years | 2.4% | ||||||||
| 14–17 years | 3.2% | ||||||||
| >18 years | 1% | ||||||||
| Rodriguez et al. | Chile | 2012 | Undergraduates | 18–24 | Cross-sectional | Posterior pharynx and tonsils | 4% | Vaccination were exclusion criteria | |
| Moreno et al. | Colombia | 2012 | Secondary students and undergraduates | 15–21 | Cross-sectional | Oropharynx | 6.8% | Not reportedb | |
| 15 years | 0.6% | ||||||||
| 16 years | 1.1% | ||||||||
| 17 years | 0.8% | ||||||||
| 18 years | 1.2% | ||||||||
| 19 years | 1.8% | ||||||||
| 20 years | 0.9% | ||||||||
| 21 years | 0.6% | ||||||||
Most studies were determined to present a medium risk of bias, and only one was assessed as having a high risk of bias (Table 6). Of the studies conducted in Central America, all were classified as having a medium risk of bias (100%). North America studies were mostly evaluated as having a medium risk of bias (80%), with one presenting high risk (10%). Two studies conducted in South America were assessed as having a low risk of bias (25%), but most were classified as medium risk.
Quality assessment of included studies.
| Question 1: Was the sample frame appropriate to address the target population? (We assessed whether at least 50% of the target population was enrolled in the study). | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Question 2: Were study participants sampled in an appropriate way?(If the sample was randomized, we considered it as ‘Yes’). | ||||||||||
| Question 3: Was the sample size adequate? (We assessed whether the authors inform if they achieved the calculated sample size). | ||||||||||
| Question 4: Were the study subjects and the setting described in detail? (We assessed whether the complete sociodemographic and epidemiological characteristics were provided, especially vaccination status). | ||||||||||
| Question 5: Was the data analysis conducted with sufficient coverage of the identified sample?(We assessed whether at least 90% of the positive samples were submitted to serogroup test). | ||||||||||
| Question 6: Were valid methods used for the identification of the condition? (We assessed whether the study used culture and/or PCR for the identification of carriers). | ||||||||||
| Question 7: Was the condition measured in a standard, reliable way for all participants?(We assessed if the samples were collected using a sterile swab). | ||||||||||
| Question 8: Was there appropriate statistical analysis?(We assessed whether the authors inform how the overall prevalence was calculated). | ||||||||||
| Question 9: Was the response rate adequate, and if not, was the low response rate managed appropriately? (We did not assess this question). | ||||||||||
| Author/Question | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Overall appraisal |
| Central America | ||||||||||
| Gutierrez et al.17 | Yes | No | No | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Martínez et al.16 | Unclear | Unclear | No | No | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Martínez et al.18 | Yes | No | No | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Núñez et al.19 | Unclear | Unclear | No | No | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Valdés et al.20 | No | No | No | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| North America | ||||||||||
| Harrison et al.23 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | Low risk of bias |
| Breakwell et al.21 | No | No | No | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Dull et al.22 | Yes | No | No | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Espinosa de los Monteros et al.30 | Yes | No | No | No | Yes | Yes | Yes | No | N/A | Medium risk of bias |
| Kellerman et al.24 | Yes | No | Unclear | No | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Knudtson et al.25 | No | No | Yes | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| McNamara et al.26 | Yes | No | No | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Patrick et al.29 | Yes | No | No | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Soeters et al.27 | Yes | No | No | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Wu et al.28 | No | No | No | No | Yes | Yes | Yes | Unclear | N/A | High risk of bias |
| South America | ||||||||||
| Nunes et al.15 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | N/A | Low risk of bias |
| Rodriguez et al.36 | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | N/A | Low risk of bias |
| Coch Gioia et al.32 | Unclear | Unclear | Yes | No | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| de Moraes et al.31 | No | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Diaz et al.35 | No | No | Yes | Yes | Unclear | Yes | Yes | Yes | N/A | Medium risk of bias |
| Moreno et al.37 | No | No | Yes | No | No | Yes | Yes | Yes | N/A | Medium risk of bias |
| Safadi et al.33 | No | No | Yes | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
| Weckx et al.34 | No | No | Yes | Yes | Yes | Yes | Yes | Yes | N/A | Medium risk of bias |
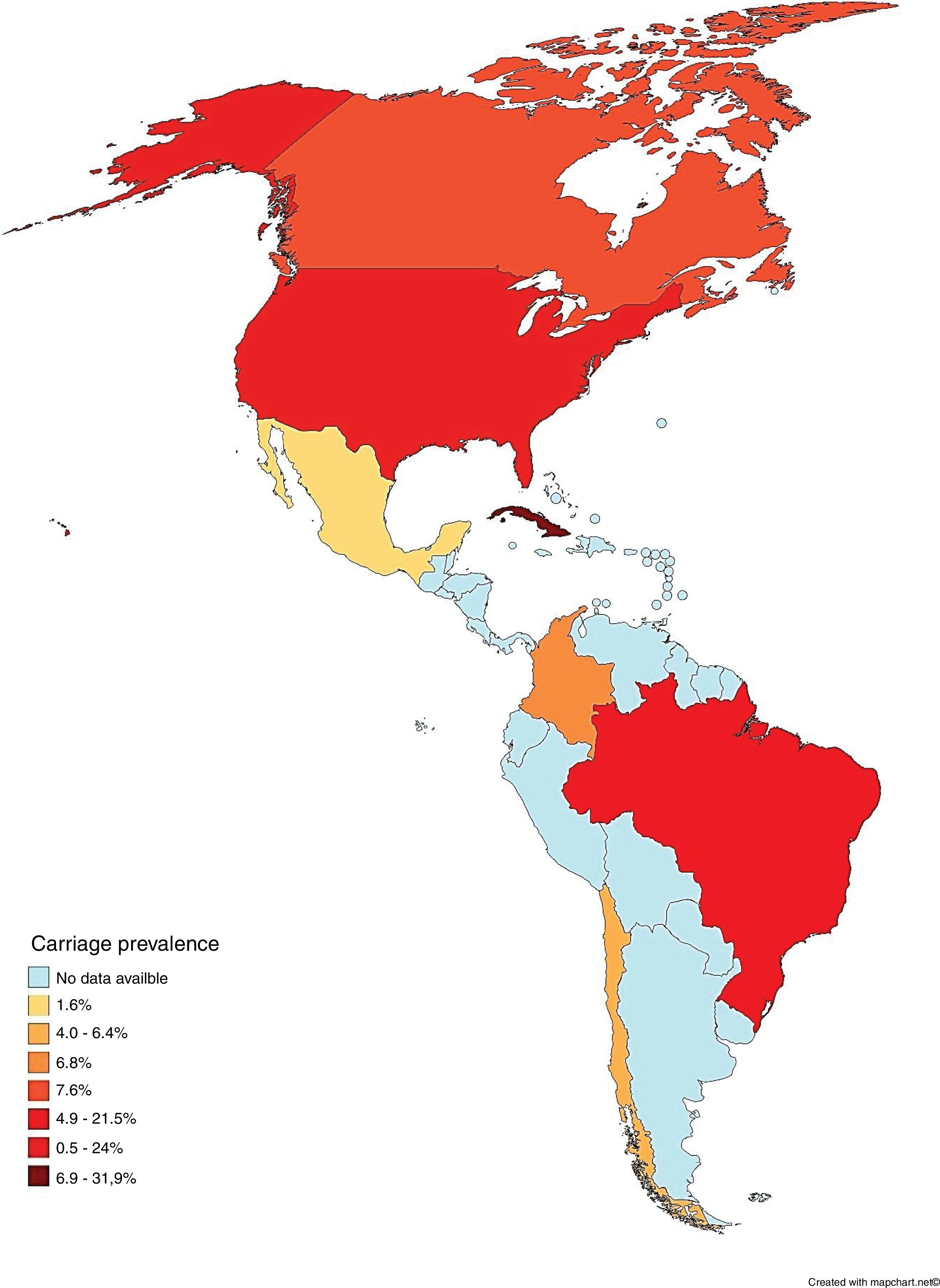
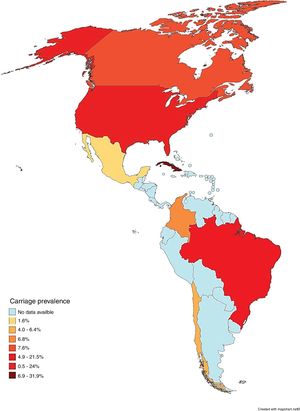
To the best of our knowledge, this systematic review was the first attempt to describe the prevalence of asymptomatic carriers of N. meningitidis throughout the Americas. The majority (78.3%; n = 18/23) of the populations studied consisted of secondary level students and undergraduates, including 19-year-olds, which is the age considered to represent the peak of carriage.38 It was also observed that carriage prevalence varied among countries, both on the same subcontinent and among different subcontinents, as shown in Fig. 2.
Several studies included semi-closed populations as the object of study, such as undergraduate students,17,19,21,26–28,30,35–37 which are considered groups presenting a high-risk of carriage.39,40
It is important to point out that the variable carrier rates and risk factors identified among selected population groups may not be reflective of the overall situation in the local populations studied.1 For example, studies conducted during outbreaks26,27,29,33 may have impacted their respective reported prevalence rates.
Articles focusing on students presented high rates of carriage prevalence,17,19,20,21,26,27 corroborating studies with similar designs carried out in other countries (10.4% in Greece41 and 12.1% in Italy).42 In fact, adolescents and young adults have been reported as the highest risk group for the acquisition and transmission of N. meningitidis.39 As expected, male sex was associated with carriage in several studies throughout the Americas.18,19,21,24,26–29,32,37 Furthermore, according to other studies conducted worldwide, several other risk factors, such as current exposure to cigarette smoke, attendance at parties and bars, household agglomeration condition, upper respiratory infection, oral sex, were also observed to increase the odds of being a carrier in adolescents and young adults.21,23,25–27,37,40,43
In Brazil, due to the increased prevalence of meningococcal C disease cases, the meningococcal serogroup C conjugate vaccine (MCC) was introduced into the routine infant vaccine schedule in 2010. This vaccine is administered at three and five months, with a booster dose scheduled at 12 months of age.44 The introduction of MCC has greatly contributed to invasive meningococcal disease control in several countries, leading to consequent effects in the carriage and transmission of meningococcal C. These effects can be attributed to both high vaccine effectiveness (direct protection), as well as to herd protection (indirect protection).10,45 Particularly in Salvador, Brazil, the low prevalence of meningococcal C (MenC) carriage observed among individuals aged 11–19 years in 2014 was associated with a catch-up campaign in 2010 that targeted adolescents and young adults in this city.15 The resulting prevalence found in Salvador was around 4–5% lower than that reported by other studies conducted in two other Brazilian cities, which did not implement a booster vaccination campaign in older age-groups.31–34
Reduced meningococcal carriage as a consequence of vaccination campaigns directed at specific serogroups was observed in a study conducted in Cuba.18 The vaccine against serogroups B and C (VA-MENGOC-BC®) was implemented in the National Immunization Program in 1991,46 which could explain the low prevalence of MenC and a high rate of non-groupable strains in subsequent studies.16,17,18,19,20 On the other hand, a significant rate of meningococcal B isolates were detected among the studies conducted in Cuba, and one study reported a higher prevalence than expected in this serogroup, which could be explained by the work activities of the population studied, involved in the manipulation of meningococcal B (MenB) strains.18
In North America, most studies were conducted in the United States to evaluate carriage prevalence and the impact of vaccination on N. meningitidis transmission and colonization.24,26,29,30 Similar to studies performed in other countries, a high prevalence of non-groupable and a low prevalence of groupable strains was found. In addition, some studies evaluating populations vaccinated against MenB and serogroups A, C, W and Y (MenACWY) were unable to determine whether meningococcal carriage and/or acquisition was reduced.21,23,26,27 This stands in contrast to the effectiveness derived from MenC vaccines reported in studies conducted in the UK and Brazil, for example.10,44,45
Some differences with respect to the methodology utilized in the included studies were observed, which seem to justify the lack of comprehensive information found in the articles assessing meningococcal carriage. We identified few studies involving individuals of more advanced age in the Americas, who are also frequently affected by IMD; hence, more studies encompassing this age group are needed. In contrast, most of the studies evaluated herein employed convenience sampling as opposed to a randomized selection method, which often unreliably represents the studied population. Although this may have interfered with the results presented by these studies, their findings nonetheless corroborated the data in the literature as a whole, which diminishes the probability of bias due to sample selection procedures.
This review is limited by the fact that searches were conducted in just four databases, and only articles were included; therefore, some papers published in annals and other sources may not have been included.
ConclusionAlthough most of the studies included herein were determined to have a medium risk of bias, the present review attempted to provide a general overview of serogroup distribution correlated with different vaccination programs specific to each country, as well as to identify relevant risk factors among N. meningitidis carriers throughout the Americas. This research was designed to support epidemiological surveillance and prevention measures to control the transmission of meningococci in a variety of populations and countries.
Conflicts of interestThe authors declare no conflicts of interest.