Since healthcare-associated pneumonia (HCAP) is heterogeneous, clinical characteristics and outcomes are different from region to region. There can also be differences between HCAP patients hospitalized in secondary or tertiary hospitals. This study aimed to evaluate the clinical characteristics of HCAP patients admitted into secondary community hospitals.
MethodsThis was a retrospective study conducted in patients with HCAP or community-acquired pneumonia (CAP) hospitalized in two secondary hospitals between March 2009 and January 2011.
ResultsOf a total of 303 patients, 96 (31.7%) had HCAP. 42 patients (43.7%) resided in a nursing home or long-term care facility, 36 (37.5%) were hospitalized in an acute care hospital for ≥ 2 days within 90 days, ten received outpatient intravenous therapy, and eight attended a hospital clinic or dialysis center. HCAP patients were older. The rates of patients with CURB-65 scores of 3 or more (22.9% vs. 9.1%; p=0.001) and PSI class IV or more (82.2% vs. 34.7%; p<0.001) were higher in the HCAP group. Drug-resistant pathogens were more frequently detected in the HCAP group (23.9% vs. 0.4%; p<0.001). However, Streptococcus pneumoniae was the most common pathogen in both groups. The rates of antibiotic change, use of inappropriate antibiotics, and failure of initial antibiotic therapy in the HCAP group were significantly higher. Although the overall survival rate of the HCAP group was significantly lower (82.3% vs. 96.8%; p<0.001), multivariate analyses failed to show that HCAP itself was a prognostic factor for mortality (p=0.826). Only PSI class IV or more was associated with increased mortality (p=0.005).
ConclusionsHCAP should be distinguished from CAP because of the different clinical features. However, the current definition of HCAP does not appear to be a prognostic for death. In addition, the use of broad-spectrum antibiotics for HCAP should be reassessed because S. pneumoniae was most frequently identified even in HCAP patients.
Pneumonia was traditionally classified as either community-acquired pneumonia (CAP) or hospital-acquired pneumonia (HAP), but in 2005 the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) introduced the concept of healthcare-associated pneumonia (HCAP).1
ATS/IDSA guidelines state that patients with HCAP should receive broad-spectrum empirical antimicrobial therapy directed at multidrug-resistant (MDR) pathogens associated with healthcare settings.1 This treatment strategy from the ATS/IDSA guidelines is based on the epidemiology and clinical outcomes of HCAP.2,3 That is, MDR bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) or Pseudomonas aeruginosa are isolated more frequently in patients with HCAP and the mortality rates associated with HCAP are significantly higher than for CAP in some reports.2,4,5 Therefore, treatment strategies based on this distinction between HCAP and CAP are thought to be very important as guides to the treatment of patients with pneumonia.
However, this concept has been controversial. The British Thoracic Society (BTS) guidelines state that there is no difference in the distribution of causative pathogens between patients with HCAP and elderly patients with CAP, although its definitions of HCAP are somewhat different.6 A recent prospective UK cohort study demonstrated that the increased mortality in HCAP according to the 2005 ATS/IDSA definitions was primarily related to underlying patient-related factors rather than the presence of antibiotic-resistant pathogens.7 This study did not establish a clear indication to change current prescribing practices in a UK cohort. A study from Europe has shown that the microbiological and mortality data of patients with nursing home-acquired pneumonia (the largest subgroup of HCAP) are more similar to the data of those with CAP.8 The reason for these varying results among studies may be that HCAP itself is heterogeneous and the regions or countries where studies were performed had different compositions of HCAP subgroups and different healthcare systems.
In Korea, there are limited data and no guidelines focusing on HCAP.9,10 Given that all of the studies were conducted in tertiary referral hospitals with over 1,000 beds and included relatively small numbers of patients residing in nursing homes or long-term care facilities (less than 10%), the results are likely to be biased towards more severe pneumonia or specific subgroups. Therefore, it is necessary to collect and evaluate data regarding patients with HCAP admitted to secondary community hospitals. This study aimed to clarify the differences in the clinical characteristics of patients with HCAP and CAP hospitalized in secondary hospitals. Also, the clinical utility of HCAP as a prognostic factor was investigated.
Material and methodsStudy designThis study was performed at the Kyung Hee University Hospital at Gangdong (a 600-bed hospital in Seoul, South Korea) and at the Jeju National University Hospital (a 540-bed hospital in Jeju, South Korea). These hospitals are classified as secondary community hospitals according to the Korean healthcare system.
Patients diagnosed with CAP (CAP group) or HCAP (HCAP group) who were hospitalized in these hospitals between March, 2009 and January, 2011 were evaluated. Clinical characteristics, comorbidities, severity, identified pathogens, antibiotic therapy and clinical outcomes were compared between the two groups. The severity of pneumonia in each group was determined using the CURB-65 (confusion, urea nitrogen, respiratory rate, blood pressure, age ≥ 65 years) score and the PSI (Pnemonia Severity Index). The study protocol was approved by the Ethical Review Committee of the two institutions. Informed consent was waived because of the retrospective nature of the study.
Categorization of pneumoniaPneumonia was defined as the presence of a new infiltrate on the chest radiography plus at least one of the following: fever (temperature ≥ 38.0° C) or hypothermia (temperature<35.0° C); new-onset cough with or without sputum production; pleuritic chest pain; dyspnea; or altered breath sounds on auscultation.11 Multi-lobar involvement was defined as the presence of pneumonic infiltrates in two or more lobes on chest radiograph or computed tomography.
According to the 2005 ATS/IDSA guidelines,1 the risk factors for HCAP include hospitalization for two days or more in the preceding 90 days, residence in a nursing home or extended care facility, home wound care, chronic dialysis within 30 days, and family member with MDR pathogens. In accordance with the guidelines, the HCAP group of this study included patients with any of the following: 1) residence in a nursing home or long-term care facility; 2) recent history of hospitalization in an acute care hospital for ≥ 2 days in the past 90 days; 3) recent outpatient intravenous therapy (such as antibiotic therapy or chemotherapy) or wound care within the past 30 days; 4) attendance at a hospital clinic or dialysis center in the last 30 days.1 CAP was defined as a diagnosis of pneumonia in patients who did not meet any of the criteria for HCAP.
MicrobiologyMicroorganisms in samples obtained from sputum, tracheal aspirate, bronchial alveolar lavage fluid, or blood were investigated. Sputum was defined as adequate when>25 neutrophils and<10 squamous epithelial cells seen under low-power field. For Mycoplasma pneumoniae or Chlamydia pneumoniae, serum samples were evaluated. Serum samples in which particle agglutination antibody titers were>320, or that were proven to have a four-fold or greater increase of antibody titers in paired sera, were regarded as positive. BinaxNOW® (Binax Inc. – Maine, USA) was routinely used to detect urinary antigens for Streptococcus pneumoniae. Seeplex® RV7 detection (Seegene, Inc. – Seoul, Korea) for respiratory viruses including influenza A/B virus, parainfluenzavirus, adenovirus, rhinovirus, metapneumovirus, respiratory syncytial virus and BinaxNOW® Legionella Urinary Antigen Test (Binax, Inc. – Maine, USA) for Legionella pneumophila serogroup 1 were performed according to the clinical judgment of the attending physicians. The antibiotic sensitivity of all isolates was determined using the disc diffusion method, and drug-resistant pathogens were defined as having resistance to two or more different classes of antimicrobial drugs.
Evaluation for clinical outcomesInappropriate antibiotic therapy was defined if the empirical antibiotics were not effective against the identified pathogens based on in vitro susceptibility testing. Initial treatment failure was defined as death during initial treatment or change of antibiotics from initial agents to another regimen after 48hours due to clinical instability. Intensive care unit (ICU) admission, need for mechanical ventilation, length of antibiotic therapy, length of hospital stay, and three- and 30-day in-hospital mortality rates were also compared between groups. The duration of overall survival (OS) was calculated as the time interval between admission and death due to any cause or last clinical contact.
Statistical analysisData are presented as number (%) or median (range) unless otherwise stated. Continuous variables were compared using Student's t-test for normally distributed variables and the Mann-Whitney U test for non-normally distributed variables. A univariate analysis was carried out using the chi-square test or Fisher's exact test for categorical data. The duration of OS was analyzed using the Kaplan-Meier method. The differences in OS were assessed using the log-rank test and multivariate Cox proportional hazards regression models were fit to assess associations between patient characteristics and time to death. p-values<0.05 were considered statistically significant. Analyses were performed using the Statistical Package for the Social Sciences (SPSS) network version 18.0 (SPSS – Chicago, IL).
ResultsClinical characteristicsA total of 303 patients (189 patients at Kyung Hee University Hospital at Gangdong and 114 patients at Jeju National University Hospital) were evaluated during the study period. There were 96 patients (31.7%) with HCAP and 207 (68.3%) with CAP. The median age of the HCAP group was 76 years (interquartile range (IQR) 69-82) and that of CAP group was 65 years (IQR 51-76), showing a statistically significant difference between the two groups (p<0.001). In the HCAP group, 42 patients (43.7%) resided in a nursing home or long-term care facility in the last 90 days, 36 patients (37.5%) were hospitalized in an acute care hospital for ≥ 2 days in the last 90 days, 10 patients (10.4%) received outpatient intravenous therapy within the past 30 days, and 8 patients (8.3%) attended a hospital clinic or dialysis center in the last 30 days.
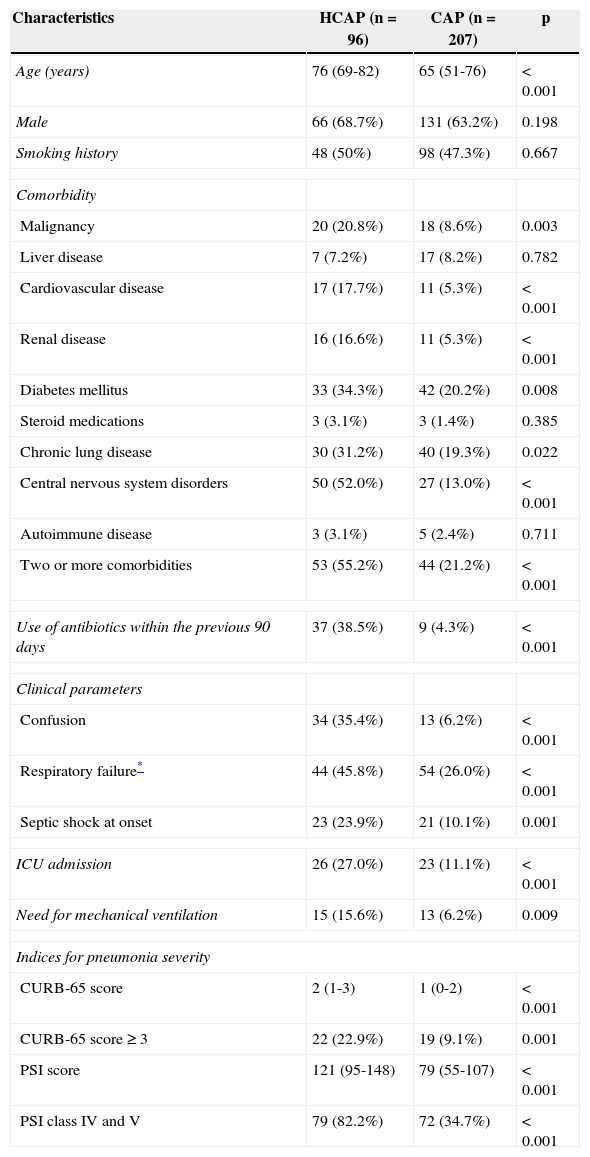
The baseline clinical features of the two groups are presented in Table 1. Comorbidities, such as malignancy, cardiovascular disease, renal disease, diabetes mellitus, chronic lung disease, and central nervous system disorders were significantly more frequent in the HCAP group than in the CAP group. The rate of patients with two or more comorbidities was higher in the HCAP group than in the CAP group (55.2% vs. 21.2%; p<0.001). The HCAP group had worse clinical parameters such as confusion, respiratory failure, and septic shock at onset than the CAP group. As a result, the rate of ICU admission and need for mechanical ventilation were significantly higher in the HCAP group. PSI and CURB-65 scores were also higher in the HCAP group. The rates of patients with CURB-65 scores of 3 or more (22.9% vs. 9.1%; p=0.001) and PSI class IV or more (82.2% vs. 34.7%; p<0.001) were higher in the HCAP group.
Baseline clinical features of patients with HCAP and CAP.
| Characteristics | HCAP (n=96) | CAP (n=207) | p |
|---|---|---|---|
| Age (years) | 76 (69-82) | 65 (51-76) | <0.001 |
| Male | 66 (68.7%) | 131 (63.2%) | 0.198 |
| Smoking history | 48 (50%) | 98 (47.3%) | 0.667 |
| Comorbidity | |||
| Malignancy | 20 (20.8%) | 18 (8.6%) | 0.003 |
| Liver disease | 7 (7.2%) | 17 (8.2%) | 0.782 |
| Cardiovascular disease | 17 (17.7%) | 11 (5.3%) | <0.001 |
| Renal disease | 16 (16.6%) | 11 (5.3%) | <0.001 |
| Diabetes mellitus | 33 (34.3%) | 42 (20.2%) | 0.008 |
| Steroid medications | 3 (3.1%) | 3 (1.4%) | 0.385 |
| Chronic lung disease | 30 (31.2%) | 40 (19.3%) | 0.022 |
| Central nervous system disorders | 50 (52.0%) | 27 (13.0%) | <0.001 |
| Autoimmune disease | 3 (3.1%) | 5 (2.4%) | 0.711 |
| Two or more comorbidities | 53 (55.2%) | 44 (21.2%) | <0.001 |
| Use of antibiotics within the previous 90 days | 37 (38.5%) | 9 (4.3%) | <0.001 |
| Clinical parameters | |||
| Confusion | 34 (35.4%) | 13 (6.2%) | <0.001 |
| Respiratory failure* | 44 (45.8%) | 54 (26.0%) | <0.001 |
| Septic shock at onset | 23 (23.9%) | 21 (10.1%) | 0.001 |
| ICU admission | 26 (27.0%) | 23 (11.1%) | <0.001 |
| Need for mechanical ventilation | 15 (15.6%) | 13 (6.2%) | 0.009 |
| Indices for pneumonia severity | |||
| CURB-65 score | 2 (1-3) | 1 (0-2) | <0.001 |
| CURB-65 score ≥ 3 | 22 (22.9%) | 19 (9.1%) | 0.001 |
| PSI score | 121 (95-148) | 79 (55-107) | <0.001 |
| PSI class IV and V | 79 (82.2%) | 72 (34.7%) | <0.001 |
Data are presented as number (%).
HCAP, healthcare-associated pneumonia; CAP, community-acquired pneumonia; ICU, intensive care unit.
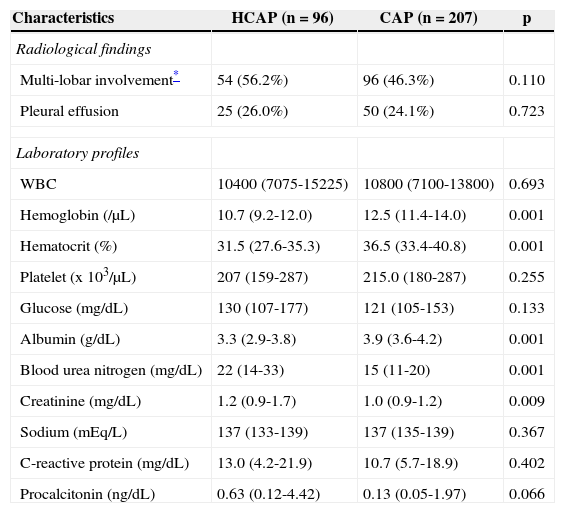
A CT scan of the lungs was obtained within three days of hospitalization for 176 patients (58.0%), including 54 (54.6%) in the HCAP group and 122 (58.9%) in the CAP group. The rates of multi-lobar involvement and pleural effusions between both groups were not significantly different (Table 2). The laboratory profiles including hemoglobin, hematocrit, albumin, blood urea nitrogen, and creatinine were worse in the HCAP group. The HCAP group showed a trend for higher PCT level compared with that of the CAP group (p=0.066).
Radiological and laboratory findings of patients with HCAP and CAP.
| Characteristics | HCAP (n=96) | CAP (n=207) | p |
|---|---|---|---|
| Radiological findings | |||
| Multi-lobar involvement* | 54 (56.2%) | 96 (46.3%) | 0.110 |
| Pleural effusion | 25 (26.0%) | 50 (24.1%) | 0.723 |
| Laboratory profiles | |||
| WBC | 10400 (7075-15225) | 10800 (7100-13800) | 0.693 |
| Hemoglobin (/μL) | 10.7 (9.2-12.0) | 12.5 (11.4-14.0) | 0.001 |
| Hematocrit (%) | 31.5 (27.6-35.3) | 36.5 (33.4-40.8) | 0.001 |
| Platelet (x 103/μL) | 207 (159-287) | 215.0 (180-287) | 0.255 |
| Glucose (mg/dL) | 130 (107-177) | 121 (105-153) | 0.133 |
| Albumin (g/dL) | 3.3 (2.9-3.8) | 3.9 (3.6-4.2) | 0.001 |
| Blood urea nitrogen (mg/dL) | 22 (14-33) | 15 (11-20) | 0.001 |
| Creatinine (mg/dL) | 1.2 (0.9-1.7) | 1.0 (0.9-1.2) | 0.009 |
| Sodium (mEq/L) | 137 (133-139) | 137 (135-139) | 0.367 |
| C-reactive protein (mg/dL) | 13.0 (4.2-21.9) | 10.7 (5.7-18.9) | 0.402 |
| Procalcitonin (ng/dL) | 0.63 (0.12-4.42) | 0.13 (0.05-1.97) | 0.066 |
Data are presented as median (interquatile range) or number (%).
HCAP, healthcare-associated pneumonia; CAP, community-acquired pneumonia; WBC, white blood cell.
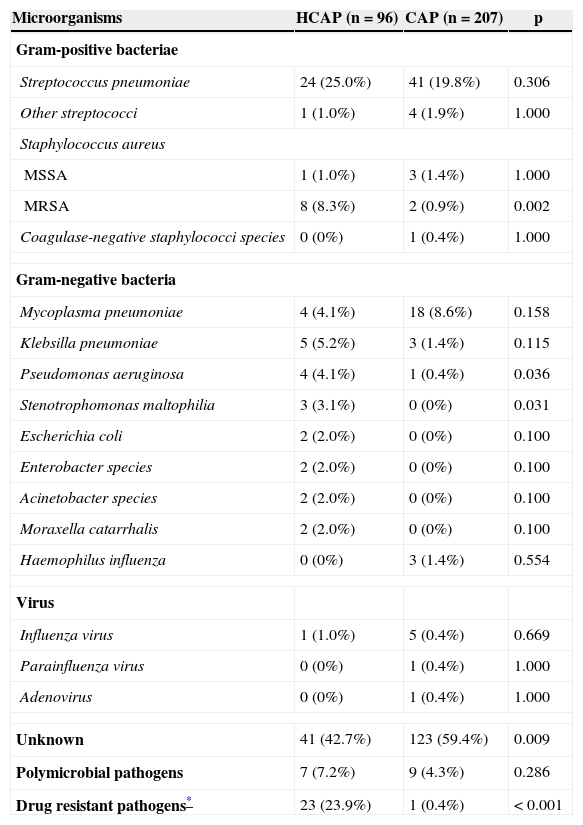
Table 3 shows the distribution of pathogens for HCAP and CAP. An etiological diagnosis was possible for 55 patients (57.3%) in the HCAP group and 84 patients (40.6%) in the CAP group. Streptococcus pneumoniae was the pathogen isolated most frequently in both groups. However, the second most common pathogens were MRSA in the HCAP group and Mycoplasma pneumoniae in the CAP group.
Microorganisms identified in patients with HCAP and CAP.
| Microorganisms | HCAP (n=96) | CAP (n=207) | p |
|---|---|---|---|
| Gram-positive bacteriae | |||
| Streptococcus pneumoniae | 24 (25.0%) | 41 (19.8%) | 0.306 |
| Other streptococci | 1 (1.0%) | 4 (1.9%) | 1.000 |
| Staphylococcus aureus | |||
| MSSA | 1 (1.0%) | 3 (1.4%) | 1.000 |
| MRSA | 8 (8.3%) | 2 (0.9%) | 0.002 |
| Coagulase-negative staphylococci species | 0 (0%) | 1 (0.4%) | 1.000 |
| Gram-negative bacteria | |||
| Mycoplasma pneumoniae | 4 (4.1%) | 18 (8.6%) | 0.158 |
| Klebsilla pneumoniae | 5 (5.2%) | 3 (1.4%) | 0.115 |
| Pseudomonas aeruginosa | 4 (4.1%) | 1 (0.4%) | 0.036 |
| Stenotrophomonas maltophilia | 3 (3.1%) | 0 (0%) | 0.031 |
| Escherichia coli | 2 (2.0%) | 0 (0%) | 0.100 |
| Enterobacter species | 2 (2.0%) | 0 (0%) | 0.100 |
| Acinetobacter species | 2 (2.0%) | 0 (0%) | 0.100 |
| Moraxella catarrhalis | 2 (2.0%) | 0 (0%) | 0.100 |
| Haemophilus influenza | 0 (0%) | 3 (1.4%) | 0.554 |
| Virus | |||
| Influenza virus | 1 (1.0%) | 5 (0.4%) | 0.669 |
| Parainfluenza virus | 0 (0%) | 1 (0.4%) | 1.000 |
| Adenovirus | 0 (0%) | 1 (0.4%) | 1.000 |
| Unknown | 41 (42.7%) | 123 (59.4%) | 0.009 |
| Polymicrobial pathogens | 7 (7.2%) | 9 (4.3%) | 0.286 |
| Drug resistant pathogens* | 23 (23.9%) | 1 (0.4%) | <0.001 |
Data are presented as number (%).
HCAP, healthcare-associated pneumonia; CAP, community-acquired pneumonia; MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphylococcus aureus.
Drug-resistant pathogens were more frequently isolated in the HCAP group (23.9% vs. 0.4%; p<0.001). MRSA, Pseudomonas aeruginosa and Stenotrophomonas maltophilia were isolated more frequently in the HCAP group than in the CAP group.
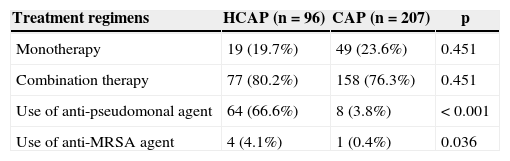
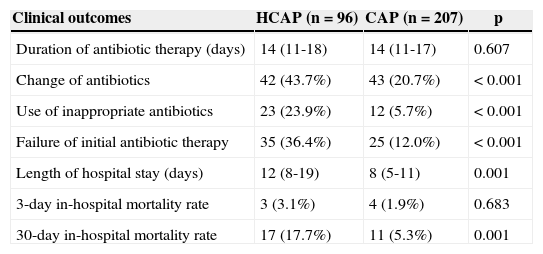
Clinical outcomesTable 4 shows the initial antimicrobial regimens. More than 75% of all patients received combination therapy. Anti-pseudomonal and/or anti-MRSA agents were used more frequently in the HCAP group (66.6% vs. 3.8%, p<0.001; 4.1% vs. 0.4%, p=0.036, respectively). Although the duration of antibiotic administration was similar for both groups (14 vs. 14 days; p=0.607), the rates of antibiotic change (43.7% vs. 20.7%; p<0.001), use of inappropriate antibiotics (23.9% vs. 5.7%; p<0.001), and failure of initial antibiotic therapy (36.4% vs. 12.0%; p<0.001) were significantly higher in the HCAP group (Table 5). Although the in-hospital mortality rate at three days was similar between the two groups, the HCAP group was hospitalized longer (p=0.001) and showed a higher in-hospital mortality rate at 30 days than the CAP group (p=0.001).
Initial antibiotic treatment.
| Treatment regimens | HCAP (n=96) | CAP (n=207) | p |
|---|---|---|---|
| Monotherapy | 19 (19.7%) | 49 (23.6%) | 0.451 |
| Combination therapy | 77 (80.2%) | 158 (76.3%) | 0.451 |
| Use of anti-pseudomonal agent | 64 (66.6%) | 8 (3.8%) | <0.001 |
| Use of anti-MRSA agent | 4 (4.1%) | 1 (0.4%) | 0.036 |
Data are presented as number (%).
HCAP, healthcare-associated pneumonia; CAP, community-acquired pneumonia; MRSA, methicillin-resistant Staphylococcus aureus.
Clinical outcomes of patients with HCAP and CAP.
| Clinical outcomes | HCAP (n=96) | CAP (n=207) | p |
|---|---|---|---|
| Duration of antibiotic therapy (days) | 14 (11-18) | 14 (11-17) | 0.607 |
| Change of antibiotics | 42 (43.7%) | 43 (20.7%) | <0.001 |
| Use of inappropriate antibiotics | 23 (23.9%) | 12 (5.7%) | <0.001 |
| Failure of initial antibiotic therapy | 35 (36.4%) | 25 (12.0%) | <0.001 |
| Length of hospital stay (days) | 12 (8-19) | 8 (5-11) | 0.001 |
| 3-day in-hospital mortality rate | 3 (3.1%) | 4 (1.9%) | 0.683 |
| 30-day in-hospital mortality rate | 17 (17.7%) | 11 (5.3%) | 0.001 |
Data are presented as number (%).
HCAP, healthcare-associated pneumonia; CAP, community-acquired pneumonia.
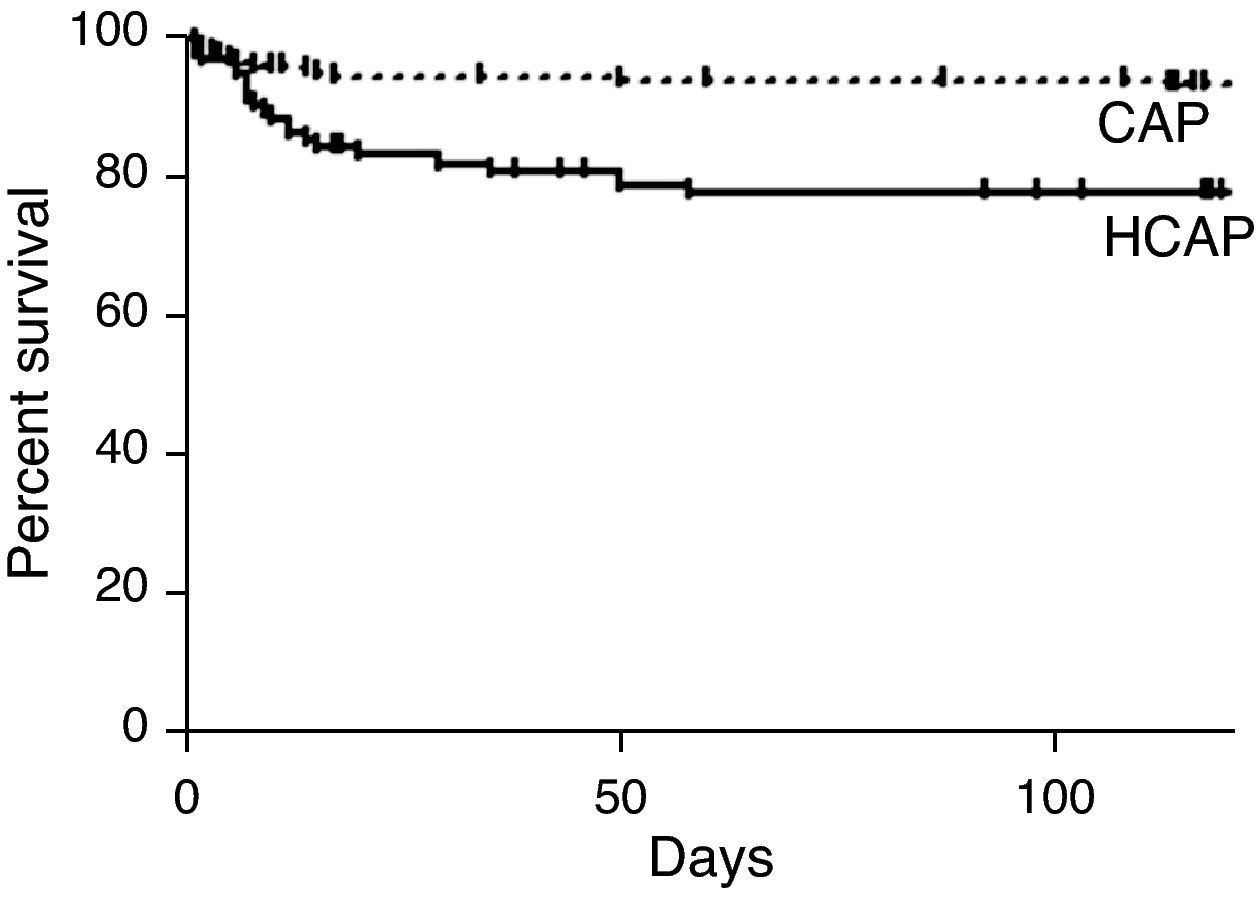
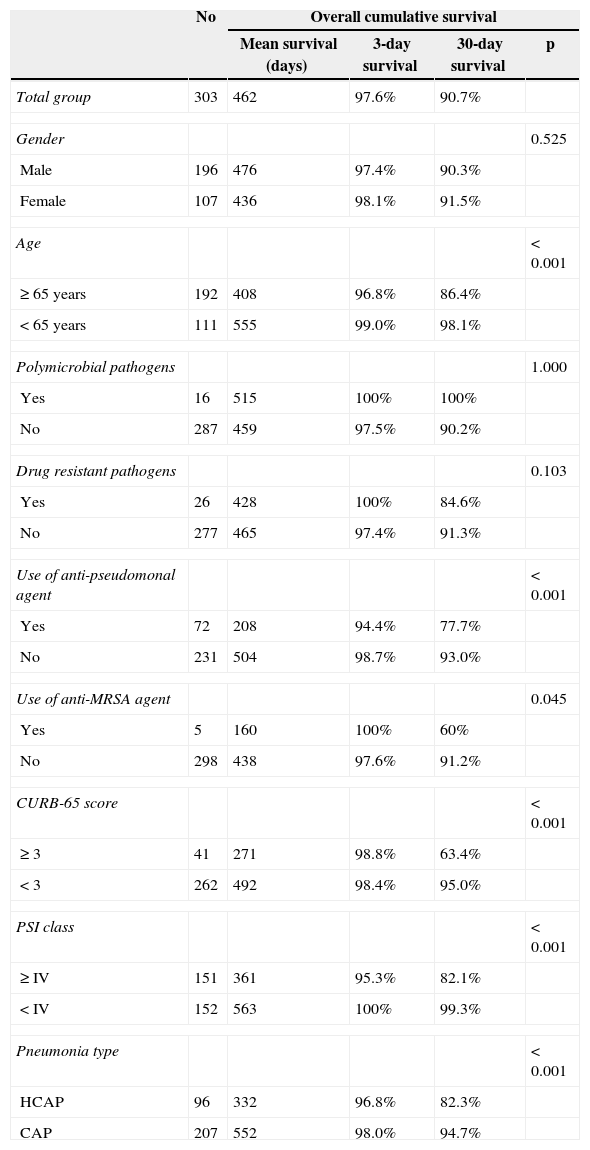
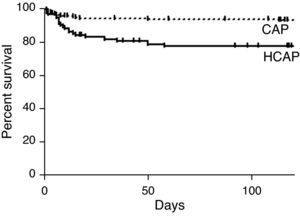
The three- and 30-day overall cumulative survival rates for all patients with pneumonia were 97.6% and 90.7%, respectively (Table 6). Notably, the OS rate of the HCAP group was significantly lower than that of the CAP group (OS rates: 82.3% vs. 96.8%, p<0.001, Fig. 1).
Univariate analysis for overall survival.
| No | Overall cumulative survival | ||||
|---|---|---|---|---|---|
| Mean survival (days) | 3-day survival | 30-day survival | p | ||
| Total group | 303 | 462 | 97.6% | 90.7% | |
| Gender | 0.525 | ||||
| Male | 196 | 476 | 97.4% | 90.3% | |
| Female | 107 | 436 | 98.1% | 91.5% | |
| Age | <0.001 | ||||
| ≥ 65 years | 192 | 408 | 96.8% | 86.4% | |
| <65 years | 111 | 555 | 99.0% | 98.1% | |
| Polymicrobial pathogens | 1.000 | ||||
| Yes | 16 | 515 | 100% | 100% | |
| No | 287 | 459 | 97.5% | 90.2% | |
| Drug resistant pathogens | 0.103 | ||||
| Yes | 26 | 428 | 100% | 84.6% | |
| No | 277 | 465 | 97.4% | 91.3% | |
| Use of anti-pseudomonal agent | <0.001 | ||||
| Yes | 72 | 208 | 94.4% | 77.7% | |
| No | 231 | 504 | 98.7% | 93.0% | |
| Use of anti-MRSA agent | 0.045 | ||||
| Yes | 5 | 160 | 100% | 60% | |
| No | 298 | 438 | 97.6% | 91.2% | |
| CURB-65 score | <0.001 | ||||
| ≥ 3 | 41 | 271 | 98.8% | 63.4% | |
| <3 | 262 | 492 | 98.4% | 95.0% | |
| PSI class | <0.001 | ||||
| ≥ IV | 151 | 361 | 95.3% | 82.1% | |
| < IV | 152 | 563 | 100% | 99.3% | |
| Pneumonia type | <0.001 | ||||
| HCAP | 96 | 332 | 96.8% | 82.3% | |
| CAP | 207 | 552 | 98.0% | 94.7% | |
HCAP, healthcare-associated pneumonia; CAP, community-acquired pneumonia; MRSA, methicillin-resistant Staphylococcus aureus.
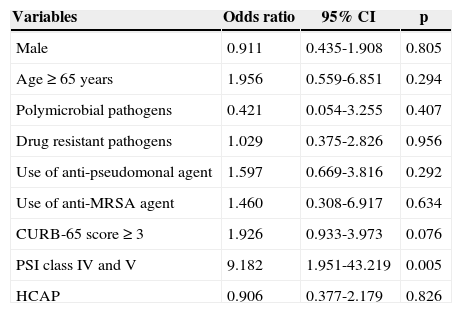
The prognostic factors associated with OS were age, drug resistant pathogen, use of anti-pseudomonal and/or anti-MRSA agents, CURB-65 score, PSI class, and designation of HCAP in univariate analysis. However, a multivariate Cox proportional hazards model for time to death showed that only PSI class IV or more was independently associated with increased mortality in patients diagnosed with pneumonia (OR: 9.182, 95% CI: 1.951-43.219, p=0.005) (Table 7). Otherwise, male gender, age, polymicrobial pathogens, drug-resistant pathogens, CURB-65 score ≥ 3, and HCAP diagnosis were not significantly associated with survival.
Cox's proportional hazard model for mortality in patients with pneumonia.
| Variables | Odds ratio | 95% CI | p |
|---|---|---|---|
| Male | 0.911 | 0.435-1.908 | 0.805 |
| Age ≥ 65 years | 1.956 | 0.559-6.851 | 0.294 |
| Polymicrobial pathogens | 0.421 | 0.054-3.255 | 0.407 |
| Drug resistant pathogens | 1.029 | 0.375-2.826 | 0.956 |
| Use of anti-pseudomonal agent | 1.597 | 0.669-3.816 | 0.292 |
| Use of anti-MRSA agent | 1.460 | 0.308-6.917 | 0.634 |
| CURB-65 score ≥ 3 | 1.926 | 0.933-3.973 | 0.076 |
| PSI class IV and V | 9.182 | 1.951-43.219 | 0.005 |
| HCAP | 0.906 | 0.377-2.179 | 0.826 |
HCAP, healthcare-associated pneumonia; CAP, community-acquired pneumonia; MRSA, methicillin-resistant Staphylococcus aureus.
Data published shortly after the establishment of the 2005 ATS/IDSA guidelines demonstrated that the spectrum of microorganisms found in HCAP was more similar to HAP than CAP.2 In particular, data from the United States has continued to confirm this finding. Thereafter, several major studies have investigated differences in the clinical characteristics of patients with HCAP and CAP.5,7,9,12–14 The findings of some recent studies have been different from those of previous studies. Therefore, some authors or experts are now questioning whether the ATS/IDSA definition of HCAP should be refined or abandoned.15 The differences in the results of these studies can be explained by differences in the subgroups of patients with HCAP and in the healthcare systems of each country.
As of 2011, a large scale, multi-center cohort study in Korea had not been performed to evaluate the clinical characteristics of HCAP. Two single-center studies were conducted in tertiary referral centers that have over 1,500 beds.9,16 These studies included a high proportion of patients who had received chemotherapy or who had a recent hospitalization, while the rate of patients residing in nursing homes was low. This patient distribution is a result of the distinct features of tertiary referral hospitals according to the healthcare system of Korea. Thus, the results of these studies cannot explain the general characteristics of patients with HCAP in Korea because they are likely to be biased toward more severe pneumonia or specific subgroups.
The average age of the population in Korea as well as in Western countries is increasing. The population above 65 years of age in Korea will be as high as 14.3% in 2019.17 This demographic feature will lead to increased numbers of the elderly residing in long-term care facilities such as nursing homes. The proportion of elderly patients with pneumonia continues to increase. Most of the elderly patients with pneumonia, either CAP or HCAP, will be hospitalized in secondary community hospitals because there are many more secondary hospitals in Korea than tertiary referral hospitals. The clinical features of patients admitted to secondary hospitals may be different from those admitted to tertiary referral hospitals. Therefore, it is important to analyze the clinical features of patients with HCAP admitted to secondary hospitals in given a region or country.
This is the first study that was performed at two secondary hospitals in Korea. The present study showed that approximately 30% of the patients with pneumonia hospitalized in a secondary hospital in Korea were diagnosed with HCAP. In terms of subgroups of HCAP, 43.7% of the patients in this study resided in a nursing home or long-term care facility, while only 10.4% had received outpatient IV therapy such as chemotherapy. This finding has epidemiological significance because the composition of the subgroups in this study was different from those of previous Korean studies.9,16 A possible reason for this finding is that HCAP itself is heterogeneous, and subgroups of HCAP can have individual clinical features.
In terms of microbiology, the most commonly identified microorganism in HCAP was Streptococcus pneumoniae, the most common bacteria of CAP. This result was also different from the results of previous Korean studies.9,16 In contrast, MRSA and Gram-negative bacteriae such as P. aeruginosa were isolated more frequently in the HCAP group. After the establishment of the 2005 ATS/IDSA guidelines for HCAP in Korea, many Korean physicians have included anti-pseudomonal and/or anti-MRSA agents into initial regimens to treat patients with HCAP. In the present study, anti-pseudomonal agents were administered to 66.6% of the patients in the HCAP group, compared with 3.8% of the patients in the CAP group (p<0.001). These results provide meaningful information. First, since there are microbiological differences between HCAP and CAP, clinicians caring for patients with HCAP should always consider drug resistant pathogens. Second, empirical regimens to cover resistant pathogens such as P. aeruginosa can be unnecessary in many patients with HCAP admitted into secondary hospitals. Although P. aeruginosa was isolated more frequently in HCAP group, its rate was low (4.1%) in our study. However, clinicians prescribed anti-pseudomonal agents to fight this organism in 66.6% of patients with HCAP. Predictive rules thought to accurately identify HCAP patients who are likely to have or not have drug-resistant pathogens should be developed. Further analyses of the subgroups of HCAP may be necessary for this purpose.
In addition to drug resistant pathogens, higher rates of inappropriate antibiotics, of failure of initial antibiotic therapy, and of 30-day mortality were observed in the HCAP group. However, multivariate analyses showed that HCAP itself was not a risk factor for mortality, and that severity indices of pneumonia such as the PSI were much more predictive of mortality. This finding underlies the fact that the current HCAP definition as a category of pneumonia, as some authors emphasize, may not be useful. However, this result may be due to the composition of the HCAP subgroups. The possibility that a specific subgroup would be a risk factor for death cannot be ruled out.
The present study has several limitations. First, since this study had a relatively small number of patients with HCAP and was performed retrospectively at two secondary Korean hospitals, it should be interpreted with caution, and its findings cannot be considered to be consistent with those of other secondary hospitals. A nationwide cohort study should be performed to overcome these limitations. Second, the ATS/IDSA 2005 guidelines recommend semi-quantitative or quantitative culture in patients with HCAP to identify causative microorganisms. However, semi-quantitative or quantitative cultures were not performed for most of the patients. Therefore, the microorganisms identified could be oropharyngeal colonizers and may not have been the definite causes of pneumonia, since sputum culture may not always distinguish between colonization and true infection despite adequate sputum collection. In spite of the limitations described above, the present study showed meaningful data regarding patients with HCAP hospitalized in secondary hospitals in Korea.
In summary, the clinical characteristics of patients with HCAP admitted into secondary hospitals in Korea are different from those with CAP. However, the current HCAP definition seems not to be associated with in-hospital mortality. In addition, because drug-sensitive S. pneumonia was identified most frequently in patients with HCAP, the use of antibiotics with extensive spectrum as recommended by the ATS/IDSA guidelines should be reassessed in the healthcare system of Korea, although patients with HCAP were more likely to receive inappropriate initial antibiotics. Also, a nationwide cohort study is needed to establish proper guidelines regarding HCAP.
Conflict of interestAll authors declare to have no conflict of interest.
Jong Hoo Lee collected and analyzed all clinical data, and made a draft of the manuscript. Yee Hyung Kim (corresponding author) planned this study, reviewed data, and revised the manuscript. This work was supported by a research grant from Jeju National University Hospital, Jeju, Korea.