Septic arthritis is an infrequent disease although very important due to the possibility of disastrous outcomes if treatment is not adequately established. Adequate information concerning the epidemiology of septic arthritis is still lacking due to the uncommon nature of the disease as well as the struggle to establish a correct case-definition.
ObjectiveTo epidemiologically characterize the population seen at Hospital das Clínicas, University of São Paulo with a diagnosis of septic arthritis between 2006 and 2011.
MethodsSixty-one patients diagnosed with septic arthritis of the knee between 2006 and 2011 were retrospectively evaluated. The patients’ clinical and epidemiological characteristics, the microorganisms that caused the infection and the patients’ treatment and evolution were analyzed.
ResultsSeptic arthritis of the knee was more common among men, with distribution across a variety of age ranges. Most diagnoses were made through positive synovial fluid cultures. The most prevalent clinical comorbidities were systemic arterial hypertension and diabetes mellitus, and the most commonly reported joint disease was osteoarthritis. Staphylococcus aureus was the prevailing pathogen. Fever was present in 36% of the cases. All patients presented elevation in inflammatory tests. Gram staining was positive in only 50.8% of the synovial fluid samples analyzed. Six patients presented complications and unfavorable evolution of their condition.
ConclusionS. aureus is still the most common pathogen in acute knee infections in our environment. Gram staining, absence of fever and normal leukocyte count cannot be used to rule out septic arthritis.
Septic arthritis of the knee is an infrequent orthopedic disease, but it is extremely important.1,2 The age group of incidence ranges from newborns to more elderly individuals. In adults, the knee is the most affected site.3–5 Even with the advances in drainage techniques and antibiotic therapy, complications such as osteomyelitis, bone erosion, joint stiffness, fibrous ankylosis, sepsis, and even death can occur.6–8 Its treatment consists of early antibiotic therapy and surgical drainage, and the common factors limiting successful treatment are lack of suspicion of the pathological condition in the initial phases of the symptoms, delayed aspiration of synovial fluid, and unsuccessful joint drainage.9–12 With increasing reports of bacteria that are resistant to antibiotics in many centers, as well as increased frequency of bacteria that were not previously associated with septic arthritis, knowledge of the epidemiological characteristics of patients in each region is fundamental for adequate therapeutic planning.13–16
ObjectiveThe objective of the present study was to epidemiologically characterize the patients with a diagnosis of septic arthritis seen at Hospital das Clínicas, Universidade de São Paulo (USP), between 2006 and 2011.
Materials and methodsThis was a retrospective study on patients with diagnosis of septic arthritis of the knee seen at the Hospital das Clínicas, Faculty of Medicine of University of São Paulo, between 2006 and 2011. All septic arthritis cases diagnosed inside the complex are referred for treatment at the Institute of Orthopedics.
The only inclusion criterion was a confirmed diagnosis of septic arthritis of the knee. The patients were selected through review of the medical records. All records of patients with IDC-10 of septic arthritis were identified and epidemiological, clinical and laboratory data were abstracted by detailed charge review, with information recorded on a standardized form. Cases of infection that occurred less than one year after any knee surgery were excluded, because these were considered post-surgical infection. Cases of multifocal septic arthritis were also excluded.
Septic arthritis was defined based on the criteria described by Newman.17 Cases were considered to be confirmed if they presented:
- -
positive synovial fluid cultures (group A);
- -
positive blood cultures with negative synovial fluid cultures (group B);
- -
negative cultures due to previous use of antibiotics, but purulent drainage of the knee joint (group C); or
- -
definite radiological or postmortem diagnosis of septic arthritis (group D).
After inclusion, the following data were gathered from the medical records: sex, age, cause of infection, origin of the patient in order to characterize the infection as community or health service-related,18 leukocyte count, defining leukocytosis as a leukocyte count greater than 11,000, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) of the blood in the initial evaluation, Gram staining, synovial fluid culturing, antibiogram, number of drainages, surgical procedures used for drainage, comorbidities, patient's immunosuppression, time elapsed between the beginning of the symptoms and drainage, previous joint disease, empirical antibiotic therapy, systemic and joint complications, and length of hospital stay.
ResultsAfter analyzing the medical records we identified 61 patients who met the inclusion criteria.
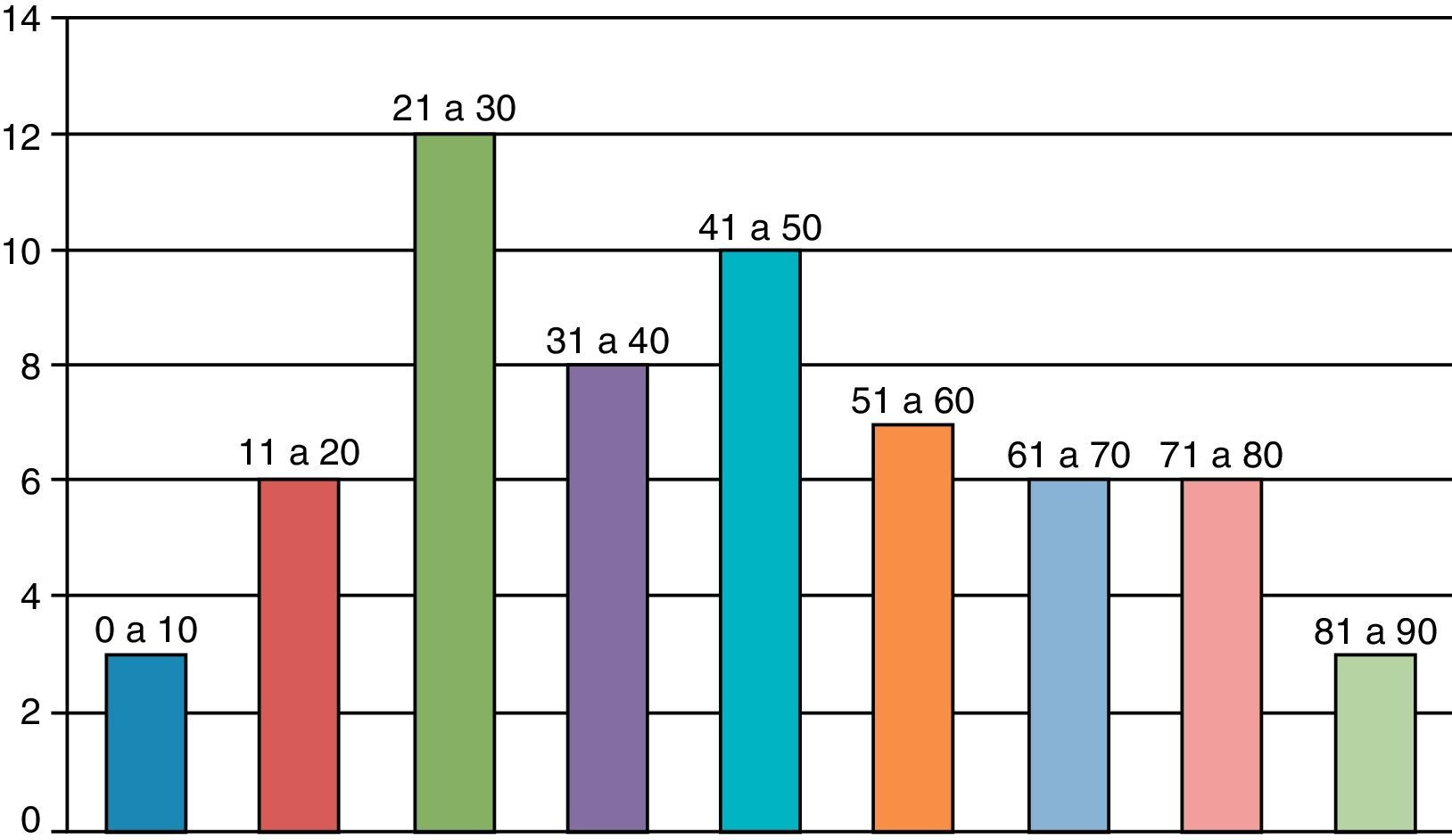
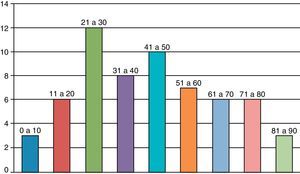
The majority were male (60% versus 40%); the mean age was 41.6 years, ranging from six months to 85 years (Fig. 1).
Out of the 61 septic arthritis cases studied, 56 had positive synovial cultures (Newman A), regardless of blood culture result. Two cases had positive blood cultures and negative synovial cultures (Newman B), and three had purulent secretion without isolation of any bacteria (Newman C). No cases were framed as Newman D.
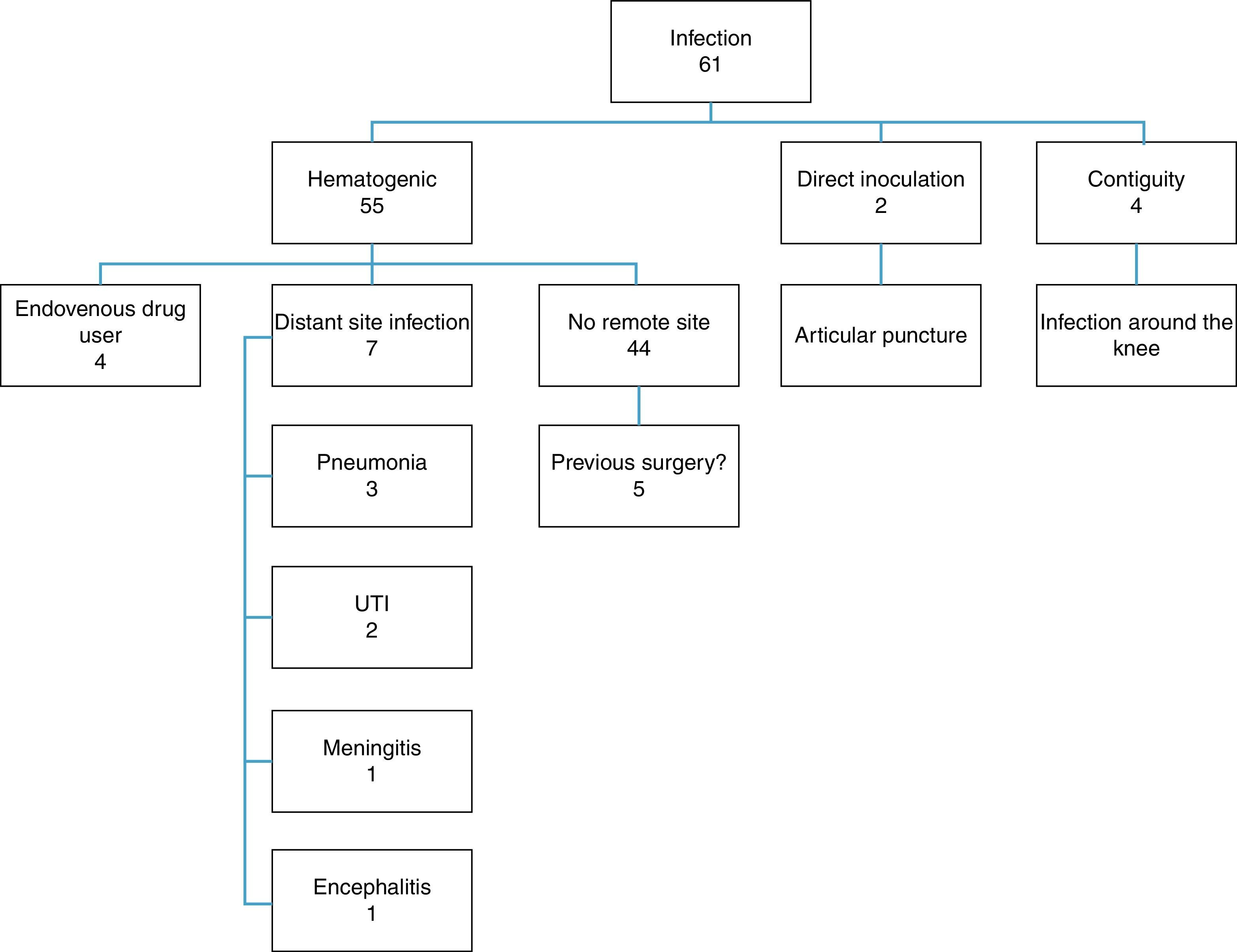
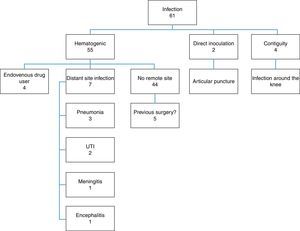
Among the patients analyzed, 55 had hematogenous infection, two had infection after knee joint aspiration (direct inoculation) and four patients were infected through soft-tissue infection around the knee (contiguity) (Fig. 2).
Previous joint disease was observed in 28 patients (45.4%), of which osteoarthritis was the most prevalent (19 cases). At least one clinical comorbidity was observed in 37 patients (60.6%), of whom 16 (26.2%) presented systemic arterial hypertension (SAH) and 12 (19.6%) diabetes mellitus (DM). In 21 patients (34.4%), immunosuppression of varying causes was observed, among which neoplasia and chronic use of corticoids were the commonest.
Leukocytosis was observed in 32 (52.4%) patients, and all patients presented altered ESR and CRP when admitted to the hospital. Temperatures above 37.8°C were observed only in 22 patients (36%). The most common complaints were pain (88.5%) and joint effusion (72.3%). The mean time elapsed between the appearance of symptoms and the clinical diagnosis was eight days. Four patients had their symptoms for more than 30 days before hospital admission.
Among the 61 patients studied, 56 had positive synovial fluid cultures (91.8%), with or without positive blood cultures. Two patients had negative cultures while bacteria were identified in blood cultures, and no bacteria were identified in three patients due to previous use of antibiotics. Gram stained bacteria were observed in only 50.8% of the cases.
Staphylococcus aureus was isolated in 44 cases (72.1%), of which 42 were from synovial cultures and two from blood cultures, and was the most prevalent bacteria. Staphylococcus epidermidis, Streptococcus spp. and Escherichia coli were the next most prevalent bacteria, with three cases each (4.9%). Salmonella spp. was found in two cases (3.2%) and Enterococcus spp., Pseudomonas aeruginosa and Neisseria gonorrhoeae in one case each (1.6%). Among the patients infected by Gram-positive bacteria, strains of oxacillin-resistant Staphylococcus spp. were isolated in 16 cases. According to the Centers for Disease Control and Prevention (CDC) criteria for oxacillin-resistant Staphylococcus spp.,19 no case was classified as community-acquired. There were no documented cases of fungi and mycobacteria.
All patients underwent open surgical drainage of the knee with access to the joint via the medial parapatellar route immediately after confirmation of the diagnosis. Forty-nine patients underwent only one surgical procedure, while 12 needed more than one drainage.
All patients received empirical antibiotic therapy for treatment until the final result from the intraoperative cultures, followed by a specific antibiotic for the bacteria identified in these cultures. Forty-two patients received oxacillin and gentamicin, 14 patients received oxacillin and ceftriaxone and five patients who were hospitalized for more than 72h due to other pathological conditions received vancomycin and cefepime. Out of the 61 patients, the causative agent was not adequately treated by empirical drugs due to resistance of the strains in 11 cases.
The mean length of hospital stay was 21 days, ranging from five to 78 days. Four patients needed care in an intensive care unit (ICU), of whom two progressed with septic shock and died while still in hospital. The other two patients went to the ICU just because of advanced age and comorbidities. One patient underwent knee disarticulation due to uncontrollable infection and soft-tissue necrosis, two patients evolved with significant stiffness of the knee (range of motion between 30 and 90°) and one patient developed chronic osteomyelitis.
Among the six patients who developed complications, five were infected by S. aureus and one by E. coli. With the exception of one of them who was HIV-positive and did not undergo the appropriate treatment, all these patients presented at least two clinical comorbidities. No previously healthy patient developed complications (Table 1).
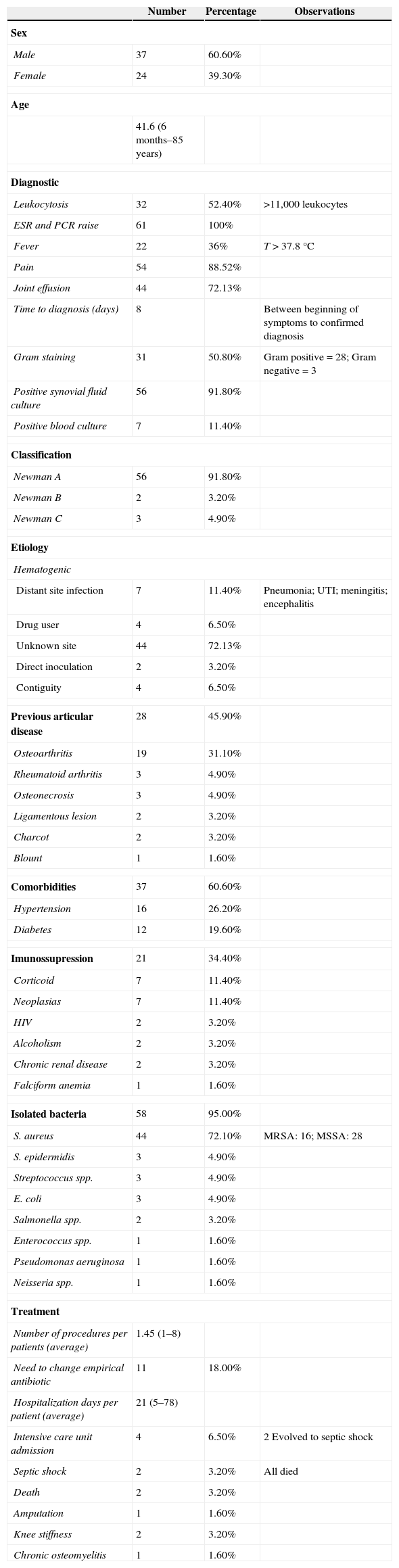
Epidemiological summary of study results.
| Number | Percentage | Observations | |
|---|---|---|---|
| Sex | |||
| Male | 37 | 60.60% | |
| Female | 24 | 39.30% | |
| Age | |||
| 41.6 (6 months–85 years) | |||
| Diagnostic | |||
| Leukocytosis | 32 | 52.40% | >11,000 leukocytes |
| ESR and PCR raise | 61 | 100% | |
| Fever | 22 | 36% | T>37.8°C |
| Pain | 54 | 88.52% | |
| Joint effusion | 44 | 72.13% | |
| Time to diagnosis (days) | 8 | Between beginning of symptoms to confirmed diagnosis | |
| Gram staining | 31 | 50.80% | Gram positive=28; Gram negative=3 |
| Positive synovial fluid culture | 56 | 91.80% | |
| Positive blood culture | 7 | 11.40% | |
| Classification | |||
| Newman A | 56 | 91.80% | |
| Newman B | 2 | 3.20% | |
| Newman C | 3 | 4.90% | |
| Etiology | |||
| Hematogenic | |||
| Distant site infection | 7 | 11.40% | Pneumonia; UTI; meningitis; encephalitis |
| Drug user | 4 | 6.50% | |
| Unknown site | 44 | 72.13% | |
| Direct inoculation | 2 | 3.20% | |
| Contiguity | 4 | 6.50% | |
| Previous articular disease | 28 | 45.90% | |
| Osteoarthritis | 19 | 31.10% | |
| Rheumatoid arthritis | 3 | 4.90% | |
| Osteonecrosis | 3 | 4.90% | |
| Ligamentous lesion | 2 | 3.20% | |
| Charcot | 2 | 3.20% | |
| Blount | 1 | 1.60% | |
| Comorbidities | 37 | 60.60% | |
| Hypertension | 16 | 26.20% | |
| Diabetes | 12 | 19.60% | |
| Imunossupression | 21 | 34.40% | |
| Corticoid | 7 | 11.40% | |
| Neoplasias | 7 | 11.40% | |
| HIV | 2 | 3.20% | |
| Alcoholism | 2 | 3.20% | |
| Chronic renal disease | 2 | 3.20% | |
| Falciform anemia | 1 | 1.60% | |
| Isolated bacteria | 58 | 95.00% | |
| S. aureus | 44 | 72.10% | MRSA: 16; MSSA: 28 |
| S. epidermidis | 3 | 4.90% | |
| Streptococcus spp. | 3 | 4.90% | |
| E. coli | 3 | 4.90% | |
| Salmonella spp. | 2 | 3.20% | |
| Enterococcus spp. | 1 | 1.60% | |
| Pseudomonas aeruginosa | 1 | 1.60% | |
| Neisseria spp. | 1 | 1.60% | |
| Treatment | |||
| Number of procedures per patients (average) | 1.45 (1–8) | ||
| Need to change empirical antibiotic | 11 | 18.00% | |
| Hospitalization days per patient (average) | 21 (5–78) | ||
| Intensive care unit admission | 4 | 6.50% | 2 Evolved to septic shock |
| Septic shock | 2 | 3.20% | All died |
| Death | 2 | 3.20% | |
| Amputation | 1 | 1.60% | |
| Knee stiffness | 2 | 3.20% | |
| Chronic osteomyelitis | 1 | 1.60% | |
Although septic arthritis is not a common disease, approximately 10 cases per year are identified at the studied hospital. Considering that these cases relate only to the knee joint, it can be said that this disease has a significant importance in our environment, in terms of incidence in relation to other joints and other regions of the world.16,20,21
The presence of patients classified as Newman type C may have caused selection bias in this study, as the evaluation of purulent secretion is arbitrary, and can sometimes be mistaken for inflammatory fluid of rheumatological diseases. However, only three patients fitted into this category.22
Considering the samples studied, the mean age demonstrates that septic arthritis is no longer a disease with great predominance among children (only three patients were younger than 10 years of age) and has gained significant representation among middle-aged and elderly patients, with 15 patients (24.5%) over 60 years of age. Although most patients with this condition have not presented unfavorable outcomes, infection among elderly patients may result in more serious consequences, considering that two patients over 60 years progressed to death.23
The great majority of the infections were of hematogenous origin. However, cases in which there was an infection in the contiguity of the joint and cases in which needles were introduced in the knee were responsible for a small portion of the infections (6.5% and 3.2%, respectively). This reinforces the notion that joint infiltrations should be carried out in appropriate environments with rigorous aseptic techniques and that infectious processes around joints should be considered severe because of the intra articular complications they can cause.24 Another risk factor for joint infections is the use of illicit intravenous drugs, because this practice provides a distant entry point that can favor hematogenic dissemination.25
The 45.9% rate of joint disease in septic arthritis patients was similar to that found in the literature, as well as greater presence of osteoarthritis among these pathological conditions.20,26 Most of the patients presented some clinical comorbidities, among which SAH and DM were the most common. Because of the patients’ advanced age and the high prevalence of SAH among this population, this condition cannot be considered to be a risk factor, unlike DM, which has been widely studied and shown to be a predisposing factor for infectious joint diseases among adults.27 Greater number of comorbidities was associated with poor prognosis of the disease. There were 34.4% of patients with immunosuppression, especially related to neoplasias and chronic use of corticosteroids. This can be explained by the fact that this hospital complex is a reference center for treatment of neoplastic and rheumatological diseases, thus concentrating a greater number of patients with these diseases or comorbidities.
Similar to cases of other infections of the musculoskeletal system, systemic symptoms such as fever were of little help for the diagnosis, since less than 50% of the patients presented with high temperature initially. Furthermore, the leukocyte count must not be used to rule out joint infection, because leukocytosis was present in only approximately 50% of the cases. Unlike the elevated leukocyte counts, inflammatory tests (ESR and CRP) were positive in 100% of the cases. Results of these tests within the normal range are not indicative of the diagnosis of septic arthritis.16,26,28
The present study did not show any increase in the number of surgical procedures, or any development of systemic or joint complications through delayed indication of surgical treatment. Only one of the patients who presented more than 10 days of complaints underwent more than one surgical drainage procedure, although there is support in the literature for the belief that a delay of more than three days after the beginning of the symptoms can lead to a worse prognosis.28,29
The great majority of the cultures showed growth of Gram-positive bacteria, especially S. aureus (72.1%), which is consistent with the epidemiology found in several regions of the world. This also corroborate the findings from our service in the 1990s, when S. aureus was found in 68.8% of the cases.30–34 Only 31 initial synovial fluid sampling were positive for Gram-stained bacteria, showing that such result cannot be taken as definitive for guiding whether to drain the joint, in cases of suspected septic arthritis.
In our service, out of the 28 cases (45.9%) that have not presented positive Gram staining, only six had not undergone drainage initially and had to wait for the definitive culture, for diagnosis confirmation. The diagnostic approach needs to be based not only on Gram staining but also on the systemic and local clinical condition, on inflammatory tests such as CRP and ESR, and on the differential cell count in the synovial fluid.
Only one patient presented infection by Neisseria, which is considered in the United States to be the most common pathogen in acute joint infections among young adults. Decreased incidence of infections by this pathogen has been observed in several centers over the last few years, although it has maintained high prevalence in the United States.34,35
High incidence of oxacillin-resistant S. aureus was observed,15,36 which prompted the initial empirical antibiotic regimen to be changed for 18% of the patients.
ConclusionIt is important to characterize each population epidemiologically, so that empirical treatment based on local evidence can be implemented. From the data analysis, it was possible to conclude that S. aureus is still the most common pathogen in acute knee infections in our environment. Risk factors such as comorbidities, immunosuppression and degenerative and inflammatory diseases also constitute important associated factors. Gram staining, absence of fever and normal leukocyte count cannot be used to rule out septic arthritis.
Conflicts of interestThe authors declare no conflicts of interest.