Schistosomiasis, a neglected tropical disease of poverty ranks second among the most widespread parasitic disease in various nations in sub-Saharan Africa. Neglected tropical diseases are causes of about 534,000 deaths annually in sub-Saharan Africa and an estimated 57 million disability-adjusted life-years are lost annually due to the neglected tropical diseases. The neglected tropical diseases exert great health, social and financial burden on economies of households and governments. Schistosomiasis has profound negative effects on child development, outcome of pregnancy, and agricultural productivity, thus a key reason why the “bottom 500 million” inhabitants of sub-Saharan Africa continue to live in poverty. In 2008, 17.5 million people were treated globally for schistosomiasis, 11.7 million of those treated were from sub-Saharan Africa. This enervating disease has been successfully eradicated in Japan, as well as in Tunisia. Morocco and some Caribbean Island countries have made significant progress on control and management of this disease. Brazil, China and Egypt are taking steps towards elimination of the disease, while most sub-Saharan countries are still groaning under the burden of the disease. Various factors are responsible for the continuous and persistent transmission of schistosomiasis in sub-Saharan Africa. These include climatic changes and global warming, proximity to water bodies, irrigation and dam construction as well as socio-economic factors such as occupational activities and poverty. The morbidity and mortality caused by this disease cannot be overemphasized. This review is an exposition of human schistosomiasis as it affects the inhabitants of various communities in sub-Sahara African countries. It is hoped this will bring a re-awakening towards efforts to combat this impoverishing disease in terms of vaccines development, alternative drug design, as well as new point-of-care diagnostics.
Human schistosomiasis otherwise called bilharzia, is a freshwater snail transmitted intravascular debilitating disease resulting from infection by the parasitic dimorphic Schistosoma trematode worms, which lives in the bloodstream of humans.1,2 The World Health Organization (WHO) regards the disease as a neglected tropical disease, with an estimated 732 million persons being vulnerable to infection worldwide in renowned transmission areas.3 Steinmann and co-workers documented that over 200 million individuals from Africa, Asia, and South America are infected with this disease.1 The WHO further estimated that schistosome infections and geohelminths accounts for over 40% of the world tropical disease burden with the exclusion of malaria.4 Humans get infected with this disease when they make contact with water contaminated with the skin-penetrating cercariae. Prevalence of schistosomiasis, at present, is still high in sub-Saharan Africa. In 2008, 17.5 million people were treated globally for schistosomiasis, 11.7 million of those from sub-Saharan Africa only.3 Approximately 120 million individuals in sub-Saharan Africa have schistosomiasis-related symptoms while about 20 million undergo hardship as a result of chronic presentations of the disease.5
Schistosomiasis has been successfully eliminated in Japan and Tunisia. Morocco and some Caribbean Islands countries have made significant progress on controlling the disease while Brazil, China, and Egypt are taking steps towards elimination of the disease.6 Schistosomiasis is more rampant in poor rural communities especially places where fishing and agricultural activities are dominant. Domestic activities such as washing clothes and fetching water in infected water expose women and children to infection. Recreational activities like swimming and poor hygiene also make children vulnerable to schistosomiasis.3 Humans are usually infected by five species of schistosomes, namely Schistosoma mansoni, Schistosoma haematobium, Schisosoma japonicum, Schistosoma mekongi, and Schistosoma intercalatum, but the main burden of disease in sub-Saharan Africa is usually attributed to two species, namely, S. mansoni and S. haematobium and are referred to as the major human schistosomes.6
Biomphalaria snails are responsible for the transmission of S. mansoni which is the source of hepatic and intestinal schistosomiasis in places like the Arabian countries, South America, and Africa. Bulinus snails transmit S. haematobium which is the chief cause of urinary schistosomiasis in Africa and in the Arab world.2 The Biomphalaria snails comprise many species including B. alexandrina, B. sudanica, B. pfeifferi, and B. hoanomphala, while the genus Bulinus comprises the following species; B. tropicus, B. globosus, B. truncatus, B. forskalli, and B. africanus.7Schistosoma japonicum is spread by the freshwater snail Oncomelania and it is responsible for intestinal and hepatosplenic schistosomal infections in Indonesia, Peoples Republic of China, and the Phillipines. It is a zoonotic parasite infecting animals including pigs, dogs, cattle, and rodents. Some other species of schistosome are parasites of different animals, but occasionally infect humans. S. mansoni can also be found in primates and rodents but the main host are human beings.2
Matured schistosomes are usually greyish or white worms with a length of 7–20mm, having a cylindrical shape with two ending suckers, a blind digestive tract, a complex tegument, and reproductive organs. A distinguishing feature in this trematode compared with other trematodes is its existence in two sexes. The male has a gyneaphoric channel or a groove, wherein it grips the female which is usually longer and thinner. The male and female schistosomes live as permanently embraced couples in the perivesical venous plexus (in S. haematobium) or in the mesentric venous plexus (in S. mansoni and S. japonicum species). The schistosomes get nourishment from the host blood and globulins by means of anaerobic glycolysis and excrete the waste back into the body of the hosts.2 A female schistosome has the capacity to produce hundreds of eggs per day as discovered in the African species, and about thousands of eggs per day in the oriental species. The individual ovum is home to miracidium larva with cilia that produce proteolytic enzymes which aid the eggs to move either towards the lumen of the bladder or towards the host intestine.2
Subsequently, the parasites eggs are released into the faeces or urine where they remain alive for about seven days. When they get into freshwater, the miracidium is released from the egg. With the aid of chemical stimuli and light, the miracidium seeks the freshwater snail which is its intermediate host. On locating the snail, the miracidium penetrates it and undergoes asexual reproduction to produce multicellular sporocytes which develop to cercarial larvae having embryonic suckers as well as a two-branched tail.2 After 4–6 weeks of infecting the snail, the cercariae leave the snail and gyrates around for about 72h looking out for the skin of a prospective host. Once released from the snail the cercariae are instigated by light mainly during the day time. On locating a human host skin, the cercariae burrow into it, migrate into the blood through the liver and lungs and undergo transformation into schistosomula also called young worms.2
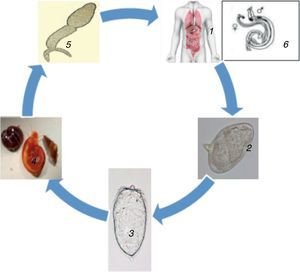
The schistosomula mature within 4–6 weeks inside the portal vein, mate, and migrate to their destination, which is either the perivesicular or mesenteric venous plexus, to start the cycle again (Fig. 1). A single infected snail has the potential of shedding thousands of cercariae daily for many months. An adult schistosome has an average lifespan of between three to five years, but it can as well live for 30 years. A single schistosome pair has a theoretical reproduction potential of up to 600 billion schistosomes.2 The intermediate freshwater snail inhabit calm or slowly moving freshwater lakes, rivers, ponds, or streams. The rate of infection in human increases with the duration of time spent in contaminated water.8 Microscopic examination of stools and urine is the gold-standard for detection (diagnosis) of schistosomal infection. The schistosome eggs are easily seen and identified on microscopy due to its peculiar size and shape, and possession of a lateral or terminal spine.8
Life cycle of Schistosoma 1. Definitive host, 2. Schistosome eggs released in urine or faeces of definitive host, 3. Free-swimming miracidia from eggs, 4. Intermediate host, 5. Cercaria (penetrates skin, loses its tail and transforms into schistosomulum), 6. Paired adult worm (schistosomulum migrates to the hepatic portal system; adult worms mature in pairs in the veins surrounding the bladder, intestines or liver. They produced eggs, the majority of which are eliminated in urine or faeces of definitive host to the environment. The eggs hatch liberating miracidia to restart the life cycle.71
Neglected tropical diseases (NTDs) are generally referred to as a collection of chronic, disabling, and physically disfiguring infectious diseases that are found mostly among poor rural dwellers and some urban population. Studies have revealed that the NTDs are more prominent in sub-Saharan African communities. NTDs have profound negative effects on children development, outcome of pregnancy, and agricultural productivity, thus a key reason why the “bottom 500 million” inhabitants of sub-Saharan Africa continue to be in poverty. This led to the concern for elimination of the NTDs as a major element of the Millennium Development Goals MDGs.9
More than 17 diseases are referred to as NTDs but seven of them have attracted more attention due to their high prevalence and resistance to control worldwide.10 NTDs poses great health as well as economic challenges for the poor dwellers of Africa, Latin America as well as Asia. These diseases are the cause of about 534,000 deaths annually; an estimated 57 million disability-adjusted life-years (DALYs) are lost annually due to the NTDs. These neglected tropical diseases put great health, social, and financial burden on economies of households and governments.11
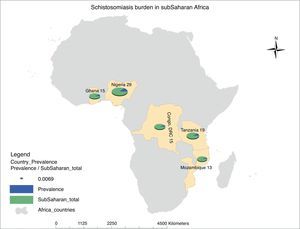
Schistosomiasis is the second most common NTDs after hookworm in Sub-Saharan Africa. Children and young adults bear most of the burden resulting from this disease in Africa. Sub-Saharan Africa accounts for 93% (192 million) of the world estimated 207 million cases of schistosomiasis. The highest prevalence of this infection is seen in Nigeria (29 million), which is closely followed by United Republic of Tanzania (19 million), Ghana, and Democratic Republic of Congo (15 million) making up the top five countries in Africa with schistosomal infection (Fig. 2). However, underestimation of the true prevalence of schistosomiasis has been reported; it is proposed that the prevalence of schistosomal-related diseases may be more than 400–600 million globally.12
Map of Africa showing ranking of estimated schistosomiasis burden in sub-Saharan African (SSA) countries. Schistosomiasis prevalence in SSA is documented to be 192 million, which is 93% of the total global prevalence of the disease. A total of 29 million people are infected by this disease in Nigeria, 19 million in Tanzania, 15 million each in Democratic Republic of Congo and Ghana, while Mozambique with 13 million people completes the top five countries with the highest prevalence in SSA. This prevalence figures by country are represented by a pie chart as a ratio of the total disease burden in SSA using a normalization figure of 0.0069. The prevalence figures are taken from Hotez and Kamath,9 while the prevalence map was generated using the ArcGIS software.
S. mansoni is the chief cause of clinical abnormalities such as hepatomegaly, splenomegaly, and periportal fibrosis in various sub-Saharan Africa countries. A study in northern Ethiopia in Alamata district revealed an alarming 73.9% prevalence of schistosomal infection, with presentation of 3.7% hepatomegaly, 7.4% splenomegaly, and 12.3% periportal fibrosis.13 A cross-sectional study carried out among 362 school age children in Machakos district in Kenya showed varying intensities of S. mansoni infections and its association with hepatomegaly. It was observed that infected children exhibited stunted growth and wasted muscles compared with children without infection.14 Cases of appendicitis-related to schistosomal infections have been reported in various studies. Schistosomal appendicitis caused by S. mansoni infection is an unusual complication in endemic communities with 0.02–6.3% prevalence, which represents 28.6% of severe appendicitis cases in such endemic regions.15
A study carried out in Nigeria reported that 2.3% of over 1000 cases of appendicitis had schistosome eggs discovered in histological sections, with 56% of the cases attributable to S. mansoni, 26.0% to S. haematobium, and 19.0% to coinfection by both species.16 In another study, 4.2% of appendicitis cases were classified as schistosomiasis of the appendix.17 Schistosomiasis due to S. mansoni is on top of the list of the causes of pulmonary hypertension worldwide, especially in areas where schistoasomiasis is endemic.18
A survey of school children aged 5–19 years in Mbita and some Islands close to Lake Victoria in Kenya revealed that the communities were highly endemic for S. mansoni infection with prevalence as high as 60.5%.19 Another survey covering the inhabitants of Lake Rweru in Rwanda indicated that 21.1% of the population screened had intestinal schistosomiasis. This suggests a likely high prevalence of schistosomiasis in the communities evaluated. Out of three communities visited, one community had a single case of infection, which could be attributed to the presence of piped water in that commmunity.20
A study carried out in Yewa North Local Government of Ogun State, Nigeria buttressed the fact that schistosomiasis exists among pregnant women. The study population consisted of pregnant women of age range 15–42 years. A prevalence of 20.8% of S. haematobium infection was observed, with the younger pregnant women at greater risk of the infection, which is in consonance with a previous report from Tanzania. It was also observed that the prevalence recorded within pregnant mothers in Yewa North was obviously lower compared with earlier reports from similar population groups due to a taboo in that part of the country restricting pregnant women from visiting natural water bodies.21
A cross-sectional study of school children aged 8–17 years in Sengerema District, Nyamatongo ward of North-West Tanzania, revealed an alarming prevalence of 64.3% S. mansoni infection. This prevalence was remarkably high in spite of previous control efforts in the ward such as mass drug administration.22 An investigative study of in-school and not-in-school children aged 6–15 years living in communities along the Tono Irrigation Canal in North Ghana revealed a prevalence of 33.2% of S. haematobium infection and a 19.8% for S. mansoni. An overall schistosomiasis prevalence of 47.7% was reported. It was also observed that there was higher infection rate in male children compared to their female counterparts, which might be due to longer period of contact with contaminated water.23
A study consisting of adult male and female subjects residing in the Volta Basin of Ghana revealed a 46.5% prevalence of urinary schiatosomiasis; this report is a good indication that schistosomal infection prevalence is not transient among adults.24 An investigative study carried out in Zenu, a surburb community of the capital city of Ghana, revealed a prevalence of 30.7% of urinary schistosomiasis among the study population of school children aged 3–16 years. Demographic analysis showed that the proximity of households to a dam was associated with the prevalence observed among the children.25 A high prevalence (57.6%) of urinary schistosomiasis and elevated intensity of S. haematobium infection (185eggs/ml) was also reported among school-age children in Niakhar District, Senegal. This endemicity could be attributed to the inhabitants’ great dependence on backwater and ponds for various domestic and recreational activities.26
The report of a pilot study in 2010, in the Eastern Cape Province of South Africa, with school-age students revealed an alarming prevalence of 73.3%. The authors observed that due to the study population size being small, there was need for further study to determine the extent of schistosomiasis in the area.27 A nation-wide survey of the prevalence of schistosomal infections and soil helminths in school children from Mozambique reported a prevalence of 47.0% S. haematobium infection and 1.0% S. mansoni infection. Further observation from this study showed that infection increases with age, with the age group 10–14 years having the highest prevalence of S. haematobium infection. It was also observed that across the districts, male children had higher prevalence compared to their female counterparts. The high prevalence of schistosomiasis was attributed to inadequate water supply in the districts, poor sanitation, and a low level of socioeconomic development in most parts of Mozambique.28
A cross-sectional study carried out in Zarima town, northwest Ethiopia among 319 elementary school children revealed a prevalence of 37.9% S. mansoni infection. The researchers observed an increased prevalence compared with previous studies in the area despite school deworming programmes. A study done at Barombi Kotto focus, South West Cameroun, revealed an alarming 69.17% prevalence of S. haematobium infection confirming high endemicity in the area. It was noted that most lakes in the area were habitats for the B. globosus intermediate host required for disease transmission.29 Added to this, another study carried out in Agboville, Cote d’Ivoire among school-aged children showed a very high prevalence of 85.3% and 53.8% for S. haematobium and S. mansoni respectively.30
Schistosomal morbidity and mortality in sub-Saharan AfricaThe significance of morbidity and mortality resulting from helminthic infections including schistosomiasis has been grossly underestimated in most developing countries.12 School-aged children, teenagers, women and young adults are mostly hit with the morbidity and mortality associated with schistosomiais.31 Population studies of schistosoma-infected children revealed that schistosomiasis can cause growth retardation, fatigue, weakness, impairment of memory and cognitive reasoning, and increased risk of anaemia, leading to poor academic performance, thus limiting the potential of infected children.8 These negative outcomes in children add to the socioeconomic burden of the society.11
Though schistosomiasis is rarely fatal, it causes long term morbidity such as anaemia which results from bleeding from the urinary and intestinal tracts due to worm invasion and movements; iron deficiency is also an outcome of the disease sequel to nutritional impairment such as nutrient malabsorption and digestive disorder like diarrhoea.32 A study in Daekena, in the Republic of Niger an area endemic for urinary schistosomiasis, revealed 41.7% of the 174 school children having iron deficiency while 57.7% exhibited anaemia related to iron deficiency.33 A longitudinal study in Burkina Faso among 1727 Burkinabe children aged 6–17 years revealed a dramatic increase in haemoglobin concentration following chemotherapy of S. haematobium compared with baseline concentration a year earlier.34
Clinical observation and autopsy strongly indicate that people, specifically elderly patients, die as a result of schistosomal-induced kidney damage.2 Urogenital schistosomiasis is a key predisposing agent for Human Immunodeficiency Virus (HIV) transmission. Urogenital schistosomiasis in HIV-infected women increases the ease of transmission to male sexual partners, as well as transmission from an HIV-infected male to his sexual partner. It also hastens the progression of the disease in people already infected with the virus by increasing the plasma concentration of the HIV RNA (viral load).35 A study among Zimbabwean women showed that women with S. haematobium eggs in their pap smear had a risk three times higher of having HIV.36
Studies on animal models and human subjects have established adverse consequences of schistosomiasis on pregnancy outcomes. S. haematobium infection has been linked to placental inflammation, leading to poor birth-outcomes as a result of placental incompetency. Heavy S. mansoni infection has also been linked to higher risk of anaemia that might subsequently lead to maternal mortality or low birth weight (LBW) babies. The anaemia may be due to urinary iron loss and faecal waste. Proinflammatory cytokines resulting from schistosomiasis infection leads to anorexia or loss of appetite of pregnant women which might ultimately result in reduced maternal weight gain and thus lead to a LBW baby.37 An observational study in Tanzania showed that there was a high prevalence of S. mansoni infection among the pregnant women and the high intensity of infection exposes the women to a higher risk of anaemia during pregnancy.38 Another study in Tanzania linked the delivery of LBW babies to infection with parasitic diseases including schistosomiasis during pregnancy.39 The study has shown an elevated progression of liver fibrosis in people co-infected with schistosomiasis and hepatitis C virus in comparison with cases of single infections, which ultimately led to advanced liver disease.40
Factors determining the continuous transmission of schistosomiasis in sub-Saharan AfricaThe continuous transmission of schistosomiasis in sub-Saharan Africa is attributable to various environmental and socio-economic factors such as:
Climatic changesThere is an established link between climatic changes and infectious disease transmission. Schistosomiasis is a typical example of diseases whose local infection and geographical expansion is influenced by climatic changes and global warming.41 Mangal and co-workers showed that a rise in ambient temperature from 20°C to 30°C will lead to over tenfold increase in the mean burden of S. mansoni infection in endemic areas. Nonetheless, at temperatures above 30°C a decrease in the disease burden was observed, probably due to higher death rate of the intermediate snail host. They observed that although an increase in disease burden leads to increased morbidity and mortality, there might be a negligible increase in prevalence of the disease.17 Rainfall patterns also have an effect on the transmission of schistosomiasis; in Senegal, the snail specie Biomphalaria pfeifferi is responsible for S. mansoni transmission during the raining season, while during the dry season S. haematobium infection is transmitted by Bilunus globosus.42
Proximity to water sourcesThe schistosome parasite requires an avenue wherein there is direct contact between the molluscan intermediate snail and the final human host for transmission of schistosomiasis to take place.43 An estimated 76% of the sub-Saharan population live close to various open water bodies which are infested with the intermediate snail host necessary for the transmission of the disease.1 Various studies have established a direct association between the intensity of the disease and proximity of infected individuals to natural water sources such as lakes, rivers, and ponds.44,45 A study carried out in Blantryre district in Malawi, showed that children whose school were closer to open water bodies had increased risk of infection,44 a finding in consonance with that reported by Clennon and co-workers.45
Man-made ecological changesEcological changes due to man-made construction of irrigation schemes, reservoirs and dams for agricultural purposes and electricity generation are also responsible for continued transmission of schistosomiasis in some in sub-Saharan African countries.46 Construction of dams led to remarkable increase in cases of urinary schistosomiasis as experienced in some sub-Saharan Africa countries such as Senegal, Cote d’Ivoire, Ghana, Mali, Namibia, and Cameroun.4 Steinmann and co-workers estimated that 13.6% (106 million) of people vulnerable to schistosomiasis reside close to irrigation schemes and large dam reservoirs.1
Socio-economic factorsSocio-economic factors influencing the continuous transmission of the debilitating disease in sub-Saharan countries include poverty occupational activities, poor sanitation and hygiene, and non-availability of potable water for domestic use.2 A World Bank analysis confirmed that the majority of the sub-Saharan population survive on between US$ 1.25–2 per day.9 King postulated a vicious cycle between poverty and schistosomiasis. He explained that poverty compels an individual to utilizing contaminated water sources for his domestic activities therefore getting infected with the disease, on getting sick due to the infection, he becomes unable to engage in activities to earn his livelihood and thus, poverty persists.12 Ugbomoiko and co-workers established the link between schistosomiasis and poverty in their cross-sectional study in two peri-urban communities in Osun State, Nigeria. An alarming 62% prevalence was recorded among 1023 individuals under study.47
An analysis by Esrey and co-workers reported on the role of improved water supply and hygiene on disease transmission and incidence. They concluded that availability of safe water and sanitation are necessary for reducing the incidence and prevalence of schistosomiasis and some other water related diseases.48 Many inhabitants of sub-Saharan countries have limited access to potable water for domestic use, leaving them with the option of using natural water bodies such as lakes, rivers, ponds, and other water sources contaminated with developmental stages of the schistosome parasite. A study carried out in northern Nigeria showed the link between safe water and intensity of urinary schistosomiasis. A higher rate of infection (88.57%) was recorded in a community that only had a pond as source of water for domestic use in comparison with 0.59% in a neighbouring community with borehole.49
Occupational activities such as fishing and farming are also risk factors for transmission of the disease. Contact with infected water is a vital factor in transmission of infection; women and children get exposed to infection during activities such as laundry, plate washing, water fetching for domestic use and bathing. Recreational activities such as swimming and diving also expose an individual to infection.3
Chemotherapy for schistosomiasis and associated falloutSeveral drugs have been used in the treatment of Schistosoma infection, and notable among them are oxamniquine, metrifonate, artemisinin derivatives, and praziquantel. Artemisinin is an antimalarial, which has been shown to have anti-schistosomal properties, with the best outcomes with artesunate and artemether. These drugs have the capacity to kill juvenile worms of both S. mansoni and S. japonicum. Trials done in China and Cote d’Ivoire established the efficacy of artemisinin derivatives. However, there is widespread concern on the use of these drugs in areas where malaria is endemic due to fear of raising Plasmodium resistance.50
Praziquantel, a pyrazinoisoquinoline derivative has been shown in randomized controlled trials to be a very safe oral drug for the treatment of schistosomiasis caused by the various schistosome species.49 This drug is made of a white crystalline bitter tasting compound which is stable in normal storage condition and insoluble in water. Commercially, the drug is available as a racemate mixture with equal ‘levo’ and ‘dextro’ isomers. The ‘levo’ part has schistosomicidal activity in vivo and in vitro. It is mainly available as 600mg crystalline tablets, but the generally recommended dosage is 40mg/kg body weight in a single dose. A 600mg/5ml syrup is also available for small children.51 Praziquantel is still the best drug for combating infections from all five species of schistosomes afflicting humans, with a cure rate of 60–90% in various epidemiological settings.52
Millions of people are given the drug yearly in sub-Saharan African nations, courtesy of various mass chemotherapy control programmes, especially under the Bill and Mellinda Gates Foundation sponsored Schistosomiasis Control Initiative (SCI).53 In the year 2002, WHO endorsed the drug safety for treating pregnant women as well as lactating mothers. However, 100% cure rates have scarcely been recorded in endemic areas.54 Praziquantel acts within an hour of oral ingestion but its actual mechanism of action on the adult worm is not yet known. Laboratory studies have revealed that the drug causes contraction of tegumental vacuoles making the worm to disengage from the wall of the veins and die. The calcium ion channel of the schistosome worm has been indirectly established as the molecular target of the drug.8 One of the hypotheses of the mode of action of this drug states that it inserts itself into the parasites membrane and produces lipid phase transition thus destabilizing the membrane.52
Praziquantel negative effects are very mild and these include nausea, vomitting, abdominal pain, malaise, and in serious infections, intense colic and bloody diarrhoea soon after treatment, which may be attributed to worm mass movement and antigen release.2 Praziquantel does not have effect on young worms both in vivo and in vitro. This significant shortcoming is responsible for poor cure rate as well as treatment failure reported in some studies, especially in areas where transmission rate is very high. To overcome this, two courses of drug administration has been advocated which gave a better cure rate.51 Extensive and intensive usage of the drug gave rise to concerns on the emergence of drug-resistant mutants of the schistosomal parasites and thus, the need for research into evolvement of new anti-schistosomal drugs.8
Schistosomiasis and cancerStudies in Africa and in the Middle East have been carried out aiming at establishing the relationship between inflammation resulting from schistosomiasis and squamous cell carcinoma of the bladder.55 The International Agency for Cancer Research in association with the World Health Organization categorized S. haematobium infection as carcinogenic. S. mansoni and S. japonicum are associated with cervical, liver, as well as colorectal carcinomas.56 Deposition of parasite ova in the bladder leads to intense inflammatory reactions that are linked to the release of oxygen-derived free radicals, which aids the production of carcinogenic N-nitrosamines, and thus malignant transformation. N-nitrosamine formation has been linked to chronic bacterial infection in the urinary bladder due to schistosomal infections.57 Groeneveld and co-workers stated in their work that eradication of schistosomiasis as well as a timely detection of bladder cancer will give room for efficient management of squamous cell carcinoma of the bladder in African patients.58
Combating schistosomiasis in sub-Saharan AfricaPreventive chemotherapeutic approach using praziquantel is the most common strategy to control schistosomiasis. It actually leads to decreased schistosomal-related morbidity but high disease re-occurrence and transmission of infection still persisted. Hence, in order to support the benefits of the drug, it must be administered at regular intervals for limitless period of time to prevent recurrency of morbidity.8 The WHO reported that as at 2010, 34.8 million people were treated with praziquantel in 30 countries; the drug was made available through yearly donation of 250 million tablets. The WHO set the goal of controlling schistosomiasis-associated morbidity in all endemic countries by the year 2020. It aimed at eliminating the disease in all endemic countries by the year 2025 and to interrupt transmission of schistosomal infections in non-endemic regions such as America, Europe, East Mediterrranean, South-East Asia, as well as some selected African countries.53
Despite sub-Saharan Africa countries having the highest burden of morbidity and mortality associated with the disease, limited efforts have been made in the control of the disease until the introduction of Schistosomiasis Control Initiatives in 2002 (SCI).59 The SCI successfully instituted national schistosomiasis control programmes in some sub-Saharan nations like Zambia, Mali, Tanzania, Burkina Faso, Niger and Uganda. However, these programmes were not successful due to incomplete vertical mass drug distribution. The coverage of the programme was ineffective, infants and preschool children were not administered the drug.46 More so, below 50.0% of the high risk population were given the drug while individuals who were not infected with the disease received the drug as the focus was on treating everyone without case finding. Thus, morbidity reduction was very low and cessation of transmission very limited.60 Before 2013, over US$ 150 million have been exhausted on schistosomiasis and other NTDs in sub-Saharan African nations, and by 2013, there was an increase after the United States of America announced a fresh funding for a initiative for combating NTDs.61
Realistically, most sub-Saharan African countries cannot afford the estimated 1.2 billion tablets of Praziquantel required to treat 400 million individuals per annum for five years at a total cost of US$ 100 million.62 Thus, schistosomiasis control in these countries cannot be achieved without foreign donor fundings.60 Over the decades, the approach used in sub-Saharan countries has aimed at reducing morbidity of infection,46 however, there is dire need for sustainable strategies geared towards transmission control and elimination of this impoverishing disease. Vaccines are known to be vital in disease control and eradication; this was evident in the 1978 eradication of small pox.63 There is a consensus among researchers in the field that effective control of transmission and long term morbidity control can be achieved through the introduction of anti-schistosomal vaccines capable of building human immune responses to parasite invasion.61,64
Improvement in water supply, sanitation and hygieneImproved sanitation and access to safe water supply are key factors in schistosomiasis control and elimination.31 Advancement in water supply and sanitation play a role in general economic development and growth of a country.65 Campbell and colleagues66 opined that there is need to progress from emphasis on treatment of morbidity due to infection towards reduction in exposure to infection through the implementation of Water, Sanitation and Hygiene (WASH) intervention policy. Many authorities in sub-Saharan Africa are not dedicated to making available clean water sources for their communities, and without accessibility to clean water, eradication of schistosomiasis in sub-Saharan Africa will continue to be a mirage.
Educating people on behavioural changesThere is a need to educate people, both young and old on the implication of voiding their bladder and faecal waste into water bodies. This behavioural change will go a long way in reducing contamination of water sources and thus lead to reduction in the rate of transmission of schistosomiasis and some other water-transmitted diseases. An intensified effort at educating people on the risk of schistosomal infection and transmission is necessary to assist in achieving positive behavioural changes regarding waste elimination and personal exposure to open water sources.9 Inhabitants of endemic areas need to be encouraged to reduce water contact as much as possible in a bid to reduce schistosomiasis transmission.67
Snail controlThough not in use in sub-Saharan Africa, the use of molluscicides reduces snail population but rarely eliminates them. This is because the snails recolonize their habitat after a while, so regular and long-term application of the chemical is required. Although, this control method was successfully used to achieve eradication of this disease in Morocco and Japan, it was also used in control programme strategies in Egypt and China. Toxicity of the chemical to fish as well as to other water-dwelling organisms is of great concern ecologically and economically. Alternatives to chemical-based molluscicides include the use of plant-based derivatives (e.g. endod) and biological control with snail competitors.31
Advances in schistosomal vaccine candidatesSub-Saharan African researchers need to be aggressively involved in the ongoing search for alternative drugs or anti-schistosomal vaccines to overcome the inherent challenges posed by this neglected but destructive disease of poverty. The foregoing are some advances in vaccine discovery. Vaccine candidates for schistosomal infections are in different phases of development. Some vaccines are in pre-clinical trial phase while others are in phase one and two clinical trials. Examples include fatty acid binding protein (FABP), Calpain, Triose-phosphate isomerase, and Tetraspanins, GST, and Paramyosin.6 The Calpain subunit called Sm-p80 has been experimentally tested for its anti-fecundity and anti-infective efficacy. Experimental mice vaccinated with DNA plasmids with Sm-p80 and interleukin-2 (IL-2) provided 59% protection, while vaccination using plasmid designed with Sm-p80 and interleukin-12 (IL-12) gave 45% protection. The study indicated that Sm-p80 is a good vaccine candidate for schistosomiasis and further advancement and optimization is needed for human trials.68 Triose-phosphate isomerase (TPI) and tetraspanins are another group of antigens with promising vaccine potential; trials have been done on buffalos to determine the efficacy levels of both vaccines on their own and fused together with heat-shock protein 70 (Hsp70). The vaccine constructs are SjCTP1-Hsp70 and SjC23-Hsp70; the former was reported to be more successful as it produced a worm burden decrease of 51.2%, liver egg decrease of 61.5%, faecal waste egg reduction of 52.1%, and 52.1% decrease in hatching faecal miracidia.69
Pearson and colleagues70 also succeeded in producing a chimeric vaccine named Sm-TSP-2. Their study indicated that this vaccine antigen could be safely used against schistosome adult worms and liver eggs as well as for the hookworm parasite. The 28kDa GST recombinant protein (Sh28GST) from S. haematobium has been experimentally tested regarding safety, immunogenicity, as well as toxicity. The vaccine candidate molecule, paramyosin (rSj97) recombinant fraction has also been discovered to give protection together with immunogenicity in BALB/c mice. The full length recombinant paramyosin protein elicited antibody responses in humans with re-infection resistance.6 However, till the moment, scientific hurdles and socioeconomic factors remain major challenges towards successful transition of the above mentioned vaccine candidates into forms that could be used for schistosomiasis intervention in sub-Saharan Africa and other regions of the world.64
ConclusionFor many decades human schistosomiasis has been of great health concern globally and mostly in communities of sub-Saharan Africa. The economic loss, disability and agony caused by the various species of schistosomes cannot be over-emphasized. Concerted efforts should be made towards the elimination of this disease of poverty in all countries in sub-Saharan Africa. The various authorities in sub-Saharan countries should make provision of clean and safe water available for the citizens. There should be constant awareness programmes to educate the population on the prominence of sanitation and hygiene in achieving disease control and the effect of schistosomiasis on the quality of life and general well-being of people.
Most importantly, it is hoped that this review will generate new interest among researchers in sub-Saharan Africa towards further scientific discoveries to combat schistosomiasis, in terms of alternative drugs to address re-infection and resistance, vaccine discovery, formulation of point-of-care diagnostics, snail control, and other germane discoveries that will serve as guide to voting-off schistosomiasis in sub-Saharan Africa and some other endemic regions of the world.
Conflicts of interestThe authors declare no conflicts of interest.
The authors would like to thank Mr Henok Solomon of the Department of Earth Sciences, University of the Western Cape, Bellville for the help received in generating the schistosomiasis burden in sub-Saharan Africa map.