Carbapenem-resistant Enterobacterales and Pseudomonas aeruginosa are being isolated from patient specimens with increasing frequency in Latin America and worldwide. The current study provides an initial description of the in vitro activity of imipenem/relebactam (IMR) against non-Morganellaceae Enterobacterales (NME) and P. aeruginosa infecting hospitalized patients in Latin America. From 2018 to 2020, 37 clinical laboratories in nine Latin American countries participated in the SMART global surveillance program and contributed 15,466 NME and 3408 P aeruginosa isolates. MICs for IMR and seven comparators were determined using CLSI broth microdilution and interpreted by CLSI M100 (2022) breakpoints. β-lactamase genes were identified in selected isolate subsets. IMR (96.9% susceptible), amikacin (95.9%), meropenem (90.7%), and imipenem (88.7%) were the most active agents against NME. Among piperacillin/tazobactam-nonsusceptible NME (n = 4124), 90.4% of isolates were IMR-susceptible (range by country, 97.2 [Chile] to 67.0% [Guatemala]) and among meropenem-nonsusceptible NME isolates (n = 1433), 74.0% were IMR-susceptible (94.1% [Puerto Rico] to 5.1% [Guatemala]). Overall, 6.3% of all collected NME isolates carried a KPC (metallo-β-lactamase [MBL]-negative), 1.8% an MBL, 0.4% an OXA-48-like carbapenemase (MBL-negative), and 0.1% a GES carbapenemase (MBL-negative). Amikacin (85.2% susceptible) and IMR (80.1%) were the most active agents against P. aeruginosa; only 56.5% of isolates were imipenem-susceptible. Relebactam increased susceptibility to imipenem by 22.0% (from 23.9% to 45.9%) in piperacillin/tazobactam-nonsusceptible isolates (n = 1031) and by 35.5% (from 5.5% to 41.0%) in meropenem-nonsusceptible isolates (n = 1128). Overall, 7.6% of all collected P. aeruginosa isolates were MBL-positive and 0.7% carried a GES carbapenemase. In conclusion, in 2018‒2020, almost all NME (97%) and most P. aeruginosa (80%) isolates from Latin America were IMR-susceptible. Continued surveillance of the in vitro activities of IMR and comparator agents against Gram-negative pathogens, and monitoring for β-lactamase changes (in particular for increases in MBLs), is warranted.
Carbapenem-resistant Enterobacterales (CRE) and carbapenem-resistant Pseudomonas aeruginosa (CRPA) are being isolated from patient specimens with increasing frequency in Latin America and worldwide.1-3 CRE and CRPA are often multidrug-resistant (MDR) and leave care providers with few safe and effective treatment options. The World Health Organization (WHO) considers CRE, extended-spectrum β-lactamases (ESBL)-producing Enterobacterales, and CRPA to be critical, priority 1 pathogens of global concern to human health.4 CRE and CRPA can result from acquired serine carbapenemases (e.g., KPC) or metallo-β-lactamases (MBLs) or from combinations of AmpC and/or ESBL expression and porin loss, upregulated efflux, and/or penicillin-binding protein mutations.1,5 Carbapenemases constitute the most frequent mechanism underlying CRE and show geographic variation in composition and prevalence.1,3,5-7 Carbapenemase carriage is much less frequent in CRPA than CRE. Carbapenem resistance in P. aeruginosa more commonly arises from derepression of PDC (Pseudomonas-derived cephalosporinase [AmpC]) together with OprD (porin) loss (for imipenem) or together with upregulation of efflux pumps such as MexAB˗OprM with or without OprD loss (for meropenem).8 New agents to treat CRE and CRPA infections continue to be needed.4 Tracking the activities of established and approved newer agents (e.g., newer β-lactam/β-lactamase inhibitor combinations) is also of critical importance.
Imipenem/relebactam (IMR) combines imipenem/cilastatin (carbapenem/renal dehydropeptidase inhibitor), and relebactam in a 2:2:1 ratio for intravenous use.9 IMR is approved in various countries for adult patients with hospital-acquired bacterial pneumonia, ventilator-associated bacterial pneumonia, complicated urinary tract infections (including pyelonephritis), and complicated intraabdominal infections caused by susceptible Gram-negative bacilli when treatment options are limited.9 Relebactam is a non-β-lactam diazabicyclooctane (DBO) inhibitor of most Ambler class A β-lactamases, including ESBLs (extended-spectrum β-lactamases) and KPCs (Klebsiella pneumoniae carbapenemases), and class C β-lactamases (AmpC).10 It restores activity to imipenem against Enterobacterales and P. aeruginosa that carry class A and class C β-lactamases and against carbapenem-resistant P. aeruginosa that arise from porin loss in combination with PDC overexpression.11
This publication is the first comprehensive summation of the SMART global surveillance program data for imipenem/relebactam tested against clinical isolates of Enterobacterales and Pseudomonas aeruginosa from Latin American countries. Our objectives were to report on the in vitro activity of IMR against non-Morganellaceae Enterobacterales (NME) and P. aeruginosa collected from hospitalized patients in Latin America, including piperacillin/tazobactam-nonsusceptible and carbapenem-nonsusceptible isolates, and to characterize β-lactamase resistance mechanisms in phenotypically resistant isolate subsets.
Materials and methodsBacterial isolatesFrom 2018 to 2020, 37 clinical laboratory sites in nine countries in the Latin America region participated in the SMART global surveillance program (Argentina, 4 sites; Brazil, 7 sites; Chile, 3 sites; Colombia, 6 sites; Ecuador, 3 sites; Guatemala, 2 sites; Mexico, 7 sites; Panama, 3 sites; Puerto Rico, 2 sites). Each site was asked to collect consecutive, clinically significant isolates of aerobic or facultatively anaerobic Gram-negative bacilli from intra-abdominal infection (IAI; 50 isolates/year), respiratory tract infection (RTI; 100 isolates/year), urinary tract infection (UTI; 50 isolates/year), and bloodstream infection (BSI; 50 isolates/year) samples. Isolates were restricted to one isolate per patient per Gram-negative species per year. Organism-specific quotas are not used in the collection of isolates by the SMART global surveillance program. All isolates were sent to IHMA (Schaumburg, IL, USA) where organism identity was confirmed using matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectrometry (Bruker Daltonics, Billerica, MA, USA) and antimicrobial susceptibility testing was performed. A total of 16,772 isolates of Enterobacterales and 3408 isolates of P. aeruginosa were collected by the 37 laboratories from 2018 to 2020. Because species in the genera Proteus, Providencia, and Morganella (Morganellaceae) demonstrate intrinsic resistance to imipenem (and IMR) by mechanisms other than by carbapenemases,12 analyses for Enterobacterales were performed using the 15,466 isolates of NME from the collection (92.2% of all Enterobacterales isolates collected). Among NME and P. aeruginosa isolates, 28.4% (n = 4400) and 59.7% (n = 2034), respectively, were collected from patients with RTI; 27.0% (n = 4171) and 12.3% (n = 419) from UTI; 21.5% (n = 3319) and 13.1% (n = 448) from IAI; 22.8% (n = 3526) and 14.5% (n = 494) from BSI; and for 0.3% of NME (n = 50) and 0.4% of P. aeruginosa isolates (n = 13), no infection source was identified. Table S1 shows the species distribution among all, piperacillin/tazobactam-nonsusceptible, and meropenem-nonsusceptible NME isolates.
Antimicrobial susceptibility testingMICs were determined by the CLSI reference broth microdilution method.13 Isolates were tested on custom-made frozen broth microdilution panels prepared at IHMA. MICs were interpreted using 2022 CLSI M100 breakpoints.12
Screening for β-lactamase genesIsolates meeting the following phenotypic criteria were screened for β-lactamase genes: imipenem- and IMR-nonsusceptible isolates of NME (excluding Serratia spp.) and P. aeruginosa; all ertapenem-nonsusceptible NME collected in 2018 only; imipenem-resistant isolates of Serratia spp. collected in 2018 only; and ceftolozane/tazobactam-nonsusceptible isolates of Enterobacterales and P. aeruginosa. Published multiplex PCR assays were used to screen for the following β-lactamase genes: ESBLs (CTX-M, GES, PER, SHV, TEM, VEB); acquired AmpC β-lactamases (ACC, ACT, CMY, DHA, FOX, MIR, MOX) and the chromosomal AmpC intrinsic to P. aeruginosa (PDC); serine carbapenemases (GES, KPC, OXA-48-like [Enterobacterales], OXA-24-like [P. aeruginosa]); and MBLs (GIM, IMP, NDM, SPM, VIM).8,14 All detected genes encoding carbapenemases, ESBLs, and PDC were amplified using gene-flanking primers and sequenced (Sanger method). For P. aeruginosa collected in 2020 only, ceftolozane/tazobactam-nonsusceptible, imipenem-nonsusceptible, and IMR-nonsusceptible isolates were characterized by short-read whole-genome sequencing (Illumina Hiseq 2 × 150 bp reads) to a targeted coverage depth of 100×15 and analyzed using the CLC Genomics Workbench (Qiagen). The Resfinder database was used to detect β-lactamase genes.16 A total of 592 NME and 65 P aeruginosa isolates collected in 2018 and 2020 (3.8% of 15,466 NME and 1.9% of 3408 P aeruginosa isolates) were not available for molecular characterization and were not included in the denominators used for carbapenemase rate calculations. This included 175 NME and 52 P aeruginosa isolates collected in Argentina, 158 NME isolates collected in Brazil, 248 NME and 13 P aeruginosa isolates collected in Colombia, and 11 NME isolates collected in Mexico. In addition, 26 randomly selected NME and 156 randomly selected P. aeruginosa isolates collected in 2020 that met the testing criteria were also not molecularly characterized (2.6% of 989 NME and 27.7% of 563 P aeruginosa isolates collected in 2020 that were available and qualified for molecular characterization). For each country, the percentage of qualified isolates collected in 2020 that were not characterized was considered when calculating carbapenemase rates.
ResultsThe most active of the eight antimicrobial agents tested against all isolates of NME were IMR (96.9% susceptible), amikacin (95.9%), meropenem (90.7%), and imipenem (88.7%) (Table 1). Greater than 96% of NME isolates from Argentina, Brazil, Chile, Colombia, Ecuador, Mexico, Panama, and Puerto Rico were IMR-susceptible; only one country, Guatemala (90.0%), had an IMR percent susceptible value < 96%. Overall, relebactam increased the susceptibility of NME isolates to imipenem by 8.2% (compared to imipenem alone) with increases ranging from 1.4% (Guatemala) to 15.1% (Puerto Rico) (Table 1). Percent susceptible values for IMR ranged from 99.2% (Escherichia coli, Citrobacter koseri) to 90.3% (Serratia marcescens) among the most common species of NME collected (Table S2) and from 97.7% (intraabdominal infection) to 95.4% (lower respiratory tract infection) among NME specimen sources (Table S3).
In vitro susceptibility of all and β-lactam-nonsusceptible isolates of non-Morganellaceae Enterobacterales collected by the SMART global surveillance program from 2018 to 2020 in Latin America.
| Phenotype Country/region (n) | % of isolates susceptible (number of susceptible isolates in P/T- and MEM-nonsusceptible isolate subsets) | |||||||
|---|---|---|---|---|---|---|---|---|
| IMR | IMI | MEM | FEP | CAZ | P/T | LVXa | AMK | |
| All isolates | ||||||||
| Argentina (1678) | 97.7 | 83.8 | 86.5 | 67.2 | 65.4 | 68.3 | 57.6 | 94.6 |
| Brazil (2477) | 96.6 | 82.7 | 86.8 | 67.6 | 68.1 | 71.1 | 60.0 | 95.7 |
| Chile (1499) | 98.5 | 94.7 | 89.4 | 60.4 | 59.0 | 66.6 | 59.7 | 95.3 |
| Colombia (2628) | 97.3 | 87.0 | 90.5 | 80.4 | 79.1 | 76.8 | 70.0 | 97.0 |
| Ecuador (1455) | 97.5 | 88.6 | 90.6 | 65.8 | 67.5 | 73.9 | 53.3 | 94.4 |
| Guatemala (1267) | 90.0 | 88.6 | 90.8 | 57.2 | 57.6 | 71.7 | 53.1 | 93.1 |
| Mexico (2612) | 96.8 | 94.8 | 96.9 | 51.0 | 50.4 | 73.4 | 47.9 | 96.8 |
| Panama (1058) | 98.5 | 95.3 | 98.4 | 79.8 | 76.7 | 86.5 | 64.0 | 98.9 |
| Puerto Rico (792) | 99.1 | 84.0 | 85.1 | 72.1 | 70.3 | 75.9 | 55.6 | 96.8 |
| Latin America (15,466) | 96.9 | 88.7 | 90.7 | 66.3 | 65.6 | 73.3 | 58.2 | 95.9 |
| P/T-nonsusceptible | ||||||||
| Argentina (532) | 95.1 (506) | 54.1 (288) | 57.5 (306) | 24.4 (130) | 17.5 (93) | 0.0 (0) | 24.1 (128) | 84.8 (451) |
| Brazil (715) | 91.2 (652) | 52.6 (376) | 54.3 (388) | 18.6 (133) | 15.5 (111) | 0.0 (0) | 21.7 (155) | 87.8 (628) |
| Chile (500) | 97.2 (486) | 87.8 (439) | 68.4 (342) | 19.0 (95) | 9.8 (49) | 0.0 (0) | 24.6 (123) | 87.4 (437) |
| Colombia (609) | 91.1 (555) | 50.2 (306) | 59.3 (361) | 44.7 (272) | 38.3 (233) | 0.0 (0) | 43.1 (261) | 88.3 (538) |
| Ecuador (380) | 91.6 (348) | 61.3 (233) | 63.9 (243) | 24.2 (92) | 20.0 (76) | 0.0 (0) | 18.9 (72) | 78.9 (300) |
| Guatemala (358) | 67.0 (240) | 65.1 (233) | 67.6 (242) | 15.1 (54) | 12.6 (45) | 0.0 (0) | 20.1 (72) | 77.1 (276) |
| Mexico (696) | 89.5 (623) | 86.4 (601) | 88.4 (615) | 21.7 (151) | 13.2 (92) | 0.0 (0) | 22.3 (155) | 91.2 (635) |
| Panama (143) | 93.7 (134) | 86.0 (123) | 88.1 (126) | 30.1 (43) | 14.0 (20) | 0.0 (0) | 21.0 (30) | 93.7 (134) |
| Puerto Rico (191) | 96.3 (184) | 35.6 (68) | 38.2 (73) | 25.1 (48) | 15.2 (29) | 0.0 (0) | 9.9 (19) | 88.0 (168) |
| Latin America (4124) | 90.4 (3728) | 64.7 (2667) | 65.4 (2696) | 24.7 (1018) | 18.1 (748) | 0.0 (0) | 24.7 (1015) | 86.5 (3567) |
| MEM-nonsusceptibleb | ||||||||
| Argentina (227) | 89.0 (202) | 2.2 (5) | 0.0 (0) | 3.1 (7) | 4.0 (9) | 0.4 (1) | 16.7 (38) | 76.2 (173) |
| Brazil (327) | 81.7 (267) | 3.7 (12) | 0.0 (0) | 1.5 (5) | 4.3 (14) | 0.0 (0) | 9.5 (31) | 82.6 (270) |
| Chile (159) | 92.5 (147) | 71.1 (113) | 0.0 (0) | 1.3 (2) | 1.9 (3) | 0.6 (1) | 3.1 (5) | 75.5 (120) |
| Colombia (249) | 78.3 (195) | 0.4 (1) | 0.0 (0) | 11.6 (29) | 9.2 (23) | 0.4 (1) | 27.8 (69) | 75.5 (188) |
| Ecuador (137) | 78.8 (108) | 1.5 (2) | 0.0 (0) | 1.5 (2) | 1.5 (2) | 0.0 (0) | 8.0 (11) | 54.7 (75) |
| Guatemala (117) | 5.1 (6) | 0.9 (1) | 0.0 (0) | 2.6 (3) | 0.9 (1) | 0.9 (1) | 12.8 (15) | 43.6 (51) |
| Mexico (82) | 19.5 (16) | 7.3 (6) | 0.0 (0) | 3.7 (3) | 4.9 (4) | 1.2 (1) | 28.0 (23) | 67.1 (55) |
| Puerto Rico (118) | 94.1 (111) | 0.8 (1) | 0.0 (0) | 3.4 (4) | 2.5 (3) | 0.0 (0) | 0.8 (1) | 83.1 (98) |
| Latin America (1433) | 74.0 (1061) | 10.0 (143) | 0.0 (0) | 3.8 (55) | 4.1 (59) | 0.3 (5) | 13.6 (195) | 73.0 (1046) |
NME, non-Morganellaceae Enterobacterales; IMR, Imipenem/Relebactam; IMI, Imipenem; MEM, Meropenem; FEP, Cefepime; CAZ, Ceftazidime; P/T, Piperacillin/Tazobactam; LVX, Levofloxacin; AMK, Amikacin.
Against the subset of piperacillin/tazobactam-nonsusceptible NME isolates (n = 4124), the percent susceptible value for IMR was 90.4% overall but highly variable by country, ranging from 97.2% susceptible for isolates from Chile to 67.0% susceptible for isolates from Guatemala; 64.7% of isolates were susceptible to imipenem (percent susceptible range by country, 87.8%‒35.6%) and 65.4% of isolates were susceptible to meropenem (88.4%‒38.2%) (Table 1). Relebactam increased the percent susceptible value to imipenem by a high of 60.7% in isolates from Puerto Rico to a low of 1.9% in isolates from Guatemala.
Against the subset of meropenem-nonsusceptible NME isolates (n = 1433), the percent susceptible value for IMR was 74.0% overall, but again, highly variable by country, ranging from 94.1% and 92.5% susceptible, respectively, for isolates from Puerto Rico and Chile to 5.1% susceptible for isolates from Guatemala (Table 1). Relebactam increased the percent susceptible value to imipenem by 64.0% overall, ranging from 93.3% in isolates from Puerto Rico, 86.8% in isolates from Argentina, but only 4.2% in isolates from Guatemala. Very low numbers of meropenem-nonsusceptible isolates (< 2% of isolates) were identified in only one country: Panama (n = 17).
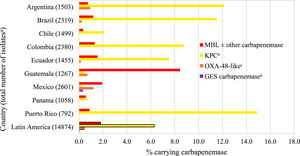
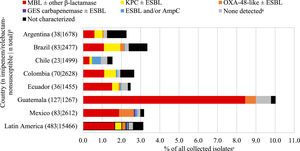
Overall, an estimated 6.3% of all collected NME isolates from Latin America carried a KPC (without a co-carried MBL), 1.8% an MBL, 0.4% an OXA-48-like carbapenemase (without other co-carried carbapenemases), and 0.1% a GES carbapenemase (without other co-carried carbapenemases) (Fig. 1, Table S4). Carbapenemase carriage rates were highest among NME isolates from Puerto Rico: 14.9% of all isolates from Puerto Rico carried a KPC and 0.9% an MBL; > 10% of NME isolates from Argentina and Brazil were also KPC-positive; < 2% of all NME isolates from Panama carried a carbapenemase of any type. There was an association between higher MBL rates and lower percent susceptible values for IMR (as well as for other β-lactams, levofloxacin, and amikacin) by country (Table 1, Fig. 1). MBLs and KPCs were identified in every country while OXA-48-like carbapenemases were not identified in Chile, Colombia, Panama, and Puerto Rico. GES carbapenemases were only identified in Mexico and only in 0.3% of NME isolates. Overall, MBLs were identified in 63.2% of molecularly characterized isolates of NME that were IMR-non-susceptible, KPC in 13.8%, OXA-48-like enzymes were present in 8.4% of IMR-non-susceptible isolates, and no acquired β-lactamases were identified in 10.1% of IMR-non-susceptible isolates (Fig. 2).
Estimated carbapenemase rates among all collected non-Morganellaceae Enterobacterales isolates by country. aExcludes 592 isolates collected in Argentina (n = 175), Brazil (n = 158), Colombia (n = 248), and Mexico (n = 11) that were not available for molecular characterization. bExcludes isolates co-carrying MBL; includes four isolates carrying OXA-48-like. cExcludes isolates co-carrying MBL and KPC. dExcludes isolates co-carrying MBL.
β-lactamase gene carriage of imipenem/relebactam-nonsusceptible non-Morganellaceae Enterobacterales isolates. Original spectrum β-lactamases (e.g., TEM-1) and intrinsic AmpC common to some NME species are not shown. aNo acquired β-lactamases detected. bOnly countries with at least 20 imipenem/relebactam-non-susceptible isolates are shown (not shown: Panama, n = 16; Puerto Rico, n = 7). cThe length of the bars represents the proportion of the imipenem/relebactam-nonsusceptible subset among all collected isolates.
Of the piperacillin/tazobactam-nonsusceptible isolates of NME that were molecularly characterized, KPC ± ESBL ± AmpC carriage (41.0% overall; from 83.6% [Puerto Rico] to 2.0% [Mexico] by country) and ESBL ± AmpC carriage (31.3% overall; from 72.1% [Chile] to 6.2% [Colombia] by country) was observed in a larger proportion of isolates than MBLs (11.8% overall), OXA-48-like enzymes (2.7%), or KPC + OXA-48-like + ESBL (0.2%); a β-lactamase mechanism was not identified in 12.6% of characterized isolates overall (Fig. S1). Among molecularly characterized isolates of NME that were meropenem-nonsusceptible, KPCs were identified in a larger proportion of isolates (63.8% overall; from 91.5% [Puerto Rico] to 4.3% [Guatemala] by country) than MBLs (19.7% overall) or OXA-48-like (1.7% overall) enzymes; a β-lactamase mechanism was not identified in only 2.7% of isolates overall (Fig. S2). KPCs were present in ≥75% of molecularly characterized meropenem-nonsusceptible NME isolates from Argentina, Brazil, Colombia, Ecuador, and Puerto Rico. MBLs were present in >60% of molecularly characterized meropenem-nonsusceptible NME isolates from Guatemala (92.2%) and Mexico (60.5%) and ≤ 15% from other countries. Isolates from Chile primarily carried ESBL ± AmpC only (80.4%).
The most active agents tested against all isolates of P. aeruginosa were amikacin (85.2% susceptible) and IMR (80.1%); all other agents tested had a percent susceptible value of approximately 74% or less (Table 2). The IMR percent susceptible value was highest in Puerto Rico (90.0%), Panama (88.3%), Argentina (86.3%), and Guatemala (86.2%) and lowest in Chile (58.1%). Overall, relebactam increased the susceptibility to imipenem of all isolates of P. aeruginosa by 23.6% compared to imipenem alone (increases ranged from 32.6% in isolates from Brazil to 12.0% in isolates from Panama), by 22.0% for all piperacillin/tazobactam-nonsusceptible isolates (n = 1031) (increases ranged from 31.0% in isolates from Brazil to 9.7% in isolates from Ecuador), and by 35.5% for all meropenem-nonsusceptible isolates (n = 1128) (increases ranged from 53.5% in isolates from Puerto Rico to 19.5% in isolates from Ecuador).
In vitro susceptibility of all and β-lactam-nonsusceptible isolates of P. aeruginosa collected by the SMART global surveillance program from 2018 to 2020 in Latin America.
IMR, Imipenem/Relebactam; IMI, Imipenem; MEM, Meropenem; FEP, Cefepime; CAZ, Ceftazidime; P/T, Piperacillin/Tazobactam; LVX, Levofloxacin; AMK, Amikacin.
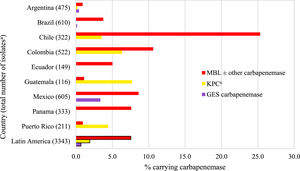
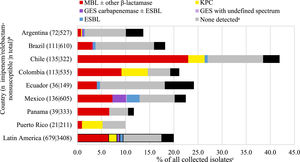
Fig. 3 and Table S5 show the estimated carbapenemase rates among all P. aeruginosa isolates. MBLs were carried by 7.6% of isolates, KPCs by 1.9%, and 0.7% carried a GES carbapenemase. MBLs were carried by 25.3%, 10.6%, and 8.6% of isolates from Chile, Colombia, and Mexico, respectively. There was a direct correlation between higher MBL rates and lower percent susceptible values for IMR by country (Table 2, Fig. 3, Table S5). KPCs were more common than MBLs in Guatemala and Puerto Rico. MBLs were identified in every country. KPC was not identified in Brazil, Ecuador, Mexico, and Panama. GES carbapenemases were most commonly seen in Mexico (3.4%). Among all molecularly characterized P. aeruginosa isolates that were IMR-nonsusceptible, MBLs were identified in 37.1% of isolates, ranging from 62.9% in Panama to 7.5% in Argentina; acquired β-lactamases were not identified in 45.8% of isolates overall, ranging from 84.9% in Argentina to 24.3% in Colombia (Fig. 4).
β-lactamase gene carriage of imipenem/relebactam-nonsusceptible P. aeruginosa isolates. Original spectrum β-lactamases (e.g., TEM-1) and intrinsic AmpC found in P. aeruginosa (PDC) are not shown. aNo acquired β-lactamases detected. bOnly countries with at least 20 imipenem/relebactam-non-susceptible isolates are shown (not shown: Guatemala, n = 16). cThe length of the bars represents the proportion of the imipenem/relebactam-nonsusceptible subset among all collected isolates.
A mechanism mediated by acquired β-lactamases was not detected in 54.2% of molecularly characterized piperacillin/tazobactam-nonsusceptible P. aeruginosa; MBLs accounted for the majority of these isolates in only Chile (53.1%) and Panama (55.6%) (Fig. S3). Similar to piperacillin/tazobactam-nonsusceptible isolates, > 50% of meropenem-nonsusceptible isolates of P. aeruginosa from most countries did not have an identifiable β-lactamase resistance mechanism (65.0% of molecularly characterized isolates overall) suggesting other mechanisms were present in most isolates (Fig. S4).
DiscussionIn 2018‒2020, 97% of 15,466 isolates of NME collected by the SMART global surveillance program from Latin American patients with IAIs, RTIs, UTIs, and BSIs were IMR-susceptible (Table 1). Among the 3% of isolates than were not susceptible to IMR, > 70% carried an MBL (63%) or an OXA-48-like carbapenemase (8%); this observation was expected given that relebactam is known to be inactive against Ambler class B (MBLs) and class D carbapenemases (e.g., OXA-48-like).11 The remainder of IMR-nonsusceptible NME isolates comprised isolates carrying KPC ± ESBL enzymes (14%), ESBL ± acquired AmpC enzymes (4%), GES (0.5%), or no β-lactamases (10%). Our data show that OXA-48-like and GES carbapenemases have a low presence in NME in Latin America. Non-β-lactamase-based resistance mechanisms (e.g., efflux, outer membrane protein changes/loss, penicillin binding protein mutation) combined with serine β-lactamases likely contributed significantly to IMR-nonsusceptible phenotypes in NME not carrying an MBL or OXA-48-like carbapenemase. Isolates of IMR-nonsusceptible NME where only KPC was identified were inferred to have also possessed other nonidentified resistance mechanisms as relebactam is a well-established inhibitor of KPC.10
The presence of both serine carbapenemases and MBLs in NME correlated with country-specific variations in percent susceptible values for piperacillin/tazobactam, meropenem, and cephalosporins (Table 1, Fig. 1). IMR percent susceptible values in NME correlated with the presence of MBLs and OXA-48-like carbapenemases. Previously, the ATLAS surveillance program published β-lactamase carriage data for carbapenem-resistant Enterobacterales and P. aeruginosa in three reports from six (2012‒2015 report17 and 2015‒2017 report18) or 10 (2017‒2019 report19) Latin American countries. Both the 2012‒2015 and 2015‒2017 reports tested isolates from Argentina, Brazil, Chile, Colombia, Mexico, and Venezuela;17,18 the 2017‒2019 report included isolates from the original six countries plus Costa Rica, Dominican Republic, Guatemala, and Panama.19 In the 2012‒2015 report, for Enterobacterales, KPCs comprised 89.1% of detected carbapenemases and MBLs were only identified in isolates from Colombia, Mexico, and Venezuela.17 By 2017‒2019, MBL-positive isolates were detected in eight of 10 Latin American countries surveyed and the proportion of carbapenemase-positive isolates that carried MBLs increased > 2-fold for isolates collected in Colombia and Venezuela (compared to the 2012‒2015 report).19 In 2017‒2019, KPC remained the most common carbapenemase identified, however, it only accounted for 61.5% of meropenem-non-susceptible Enterobacterales from all 10 countries surveyed (66.5% of isolates from the original six countries).19 MBL incidence increased in Latin American isolates over time, from 0.2% of isolates collected in 2012‒201517 to 0.6% of isolates collected in 2015‒201718 to 1.3% of isolates from the original six countries and 1.7% of isolates from all ten countries in 2017‒2019.19 In the current study, an estimated 1.8% of all NME isolates from Latin America carried an MBL. As we observed in the current study (8.4% of all NME isolates from Guatemala carried an MBL) (Fig. 1), the proportion of MBL-positive isolates collected in Guatemala in 2017‒2019 for the ATLAS study (12.9% of isolates) was much higher than observed for the nine other countries surveyed (≤ 2.2%).19 In the current study, 20% of characterized carbapenem-nonsusceptible NME were MBL-positive (Fig. S2) similar to an earlier report (19%) of Latin American isolates collected from 2016 to 2018.2
Previous surveillance studies of Latin American isolates have generally reported rates of carbapenem-resistant Enterobacterales of 5% overall, but that were higher (> 10%) for K. pneumoniae and Enterobacter spp.17-21 In the current study, approximately 90% of NME isolates were susceptible to imipenem and meropenem (Table 1). The exclusion of Proteus, Providencia, and Morganella (Morganellaceae) from the dataset, which are generally meropenem-susceptible, would be expected to increase the impact that K. pneumoniae and Enterobacter spp. isolates have on the overall rate of carbapenem resistance.
In the current study, 80% of 3408 isolates of P. aeruginosa collected by the SMART global surveillance program from Latin American patients were IMR-susceptible (Table 2). Among IMR-nonsusceptible P. aeruginosa, MBLs were identified in 37.1% of isolates; KPCs in 9.7% of isolates, ESBLs in 3.9% of isolates, and GES carbapenemases in 3.4% of isolates; acquired β-lactamases were not identified in almost half (45.8%) of all IMR-nonsusceptible isolates. Non-carbapenemase-based resistance mechanisms (e.g., OprD loss/mutation in combination with PDC derepression) likely contributed significantly to IMR-nonsusceptible phenotypes in P. aeruginosa not carrying an MBL.8 Given that imipenem is a strong inducer of AmpC (PDC) there will be high levels of PDC whenever imipenem is present, in a patient or an in vitro susceptibility test. PDC expression levels have been correlated to imipenem MICs in P. aeruginosa with defects in OprD.22 The presence or absence of an MBL correlated with country-specific variations in the activity of IMR against all, piperacillin/tazobactam-nonsusceptible, and meropenem-nonsusceptible P. aeruginosa (Table 2, Fig. 3). IMR percent susceptible values were generally higher in countries with lower levels of MBL carriage.
Previously published ATLAS surveillance studies have also reported significant numbers of MBLs in meropenem-nonsusceptible P. aeruginosa isolates. In 2017‒2019, 25.6% of meropenem-non-susceptible P. aeruginosa isolates collected in 10 Latin American countries carried MBLs (24.8% of isolates from the original six countries17 carried MBLs).19 That percentage was considerably higher (> 10%) than the 14.7% of meropenem-, doripenem-, or imipenem-non-susceptible P. aeruginosa isolates reported as MBL-positive among P. aeruginosa isolates from the original six countries in 2012‒2015.17 In the current study, 23.1% of characterized meropenem-nonsusceptible P. aeruginosa isolates carried an MBL. Previous surveillance studies of Latin American isolates have generally reported percent susceptible rates for carbapenem tested against P. aeruginosa of 60%‒70%,17-21 similar to findings in the current study (57%‒67%).
The strengths of the current study are that it collected isolates from sites in nine countries according to a consistent protocol and used reference broth microdilution antimicrobial susceptibility testing and molecular testing performed in a central laboratory. Study limitations include that it used sample quotas to collect isolates from different infection types that may affect the overall estimates of resistance and β-lactamase prevalence. The number of medical centers participating in each country did not correlate with individual country population size. Some change in study participation by individual medical centers also occurred over the three years of the study.
In conclusion, the current study provides the first report of surveillance data for IMR for Latin America from the SMART global surveillance program. In 2018‒2020, we observed 97% of NME and 80% of P. aeruginosa from Latin America were IMR-susceptible. Country-specific differences in carbapenem-resistance mechanisms do exist in Latin America and should be considered when evaluating treatment options. IMR appears to be a potential treatment option for infections caused by antimicrobial-resistant NME and P. aeruginosa when MBLs and OXA-48-like carbapenemases are absent. Increases in the prevalence of MBL-positive isolates of Gram-negative bacilli may be occurring in Latin America and will pose treatment challenges for all newer β-lactam/β-lactamase inhibitor combinations, including IMR. Continued surveillance of the in vitro activities of IMR and comparators against Gram-negative pathogens and monitoring for β-lactamase changes, particularly that of MBLs, is important.
Authors’ contributionsSHL and DFS work for IHMA, which receives funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA for the SMART global surveillance program. JAK is a consultant to IHMA. FS, JP, KY, and MRM are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and own stock in Merck & Co., Inc., Rahway, NJ, USA. The IHMA authors and JAK do not have personal financial interests in the sponsor of this paper (Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA).
Ethical approvalNot required.