Paracoccidioidomycosis is a systemic mycosis considered endemic and limited to Latin America with the majority of registered cases originating from Brazil. The purpose of this paper was to report a case of a female patient with paracoccidioidomycosis mimicking inflammatory bowel disease and to systematically review available cases of the intestinal presentation of this infectious disease.
Case reportFemale patient, 32-years old, previously asymptomatic, presenting with acute pain in the lower right abdomen, associated with signs of peritoneal irritation and abdominal distension. Urgent surgery was performed, which identified a severe suppurative perforated ileitis. The anatomopathological study revealed fungal structures shaped as a ship's pilot wheel in Grocott-Gomori's staining, suggestive of Paracoccidioides spp.
MethodsStudies were retrieved based on Medical Subject Headings and Health Sciences Descriptors, which were combined using Boolean operators. Searches were run on the electronic databases Scopus, Web of Science, MEDLINE (PubMed), BIREME (Biblioteca Regional de Medicina), LILACS (Latin American and Caribbean Health Sciences Literature), SciELO (Scientific Electronic Library Online), Embase, and Opengray.eu. Languages were restricted to English, Spanish and Portuguese. There was no date of publication restrictions. The reference lists of the studies retrieved were searched manually. Simple descriptive analysis was used to summarize the results.
ResultsOur search strategy retrieved 581 references. In the final analysis, 34 references were included, with a total of 46 case reports. The most common clinical finding was abdominal pain and weight loss present in 31 (67.3%) patients. Most patients were treated with itraconazole (41.3%) and amphotericin B (36.9%). All-cause mortality was 12.8%.
ConclusionsParacoccidioidomycosis should be suspected in endemics areas, specially as a differential diagnosis for inflammatory bowel disease. Endoscopic tests and biopsy are useful for diagnosis and treatment with antifungal drugs seem to be the first treatment option to achieve a significant success rate.
Paracoccidioidomycosis (PCM) is a systemic mycosis considered endemic and limited to Latin America with the majority of registered cases originating from Brazil.1 There are two species known to cause this disease: P. brasiliensis and P. lutzii.2
Currently, PCM is classified into two main clinical presentations including acute/subacute (juvenile type) and chronic (adult type) forms. Acute/subacute PCM is frequently observed in children and adults under 30 years old3 accounting for less than 10 percent of the cases. In this form, both men and women are affected equally. Symptoms and signs observed in this form include weight loss, fever, mild to moderate anemia, and enlargement of multiple lymph nodes. In addition, organs including the liver, spleen, bone marrow, and digestive tract may be affected.4
In contrast, the chronic form is more common and can represent a reactivation of the primary infection. It commonly affects men between 30 and 60 years of age who work in agriculture for a long time.3 PCM can disseminate to any part of the body via lymphatic or hematogenic route. In the adult-type disease, there might be pulmonary involvement and ulcerated lesions of the skin and mucosa (oral and nasal). Besides the mouth, other gastrointestinal portions are rarely affected. However, all segments may be involved. In these cases, intestinal PCM predominantly affects the jejunum, ileum, and proximal portions of the large intestine.5
PCM has been previously described as a condition that can mimick other diseases, especially Crohn's disease (CD), in patients from endemic countries.6 CD is a chronic inflammatory condition that can affect any part of the digestive tract. Clinical manifestations of gastrointestinal involvement of PCM include abdominal pain, nausea, diarrhea, and colonoscopy findings such as deep ulcers and strictures are very similar to those of Crohn's disease.7 For this reason, PCM should be considered in the differential diagnosis of inflammatory bowel diseases, specially in endemic countries, such as Brazil.8
The purpose of this paper is to report a case of a female patient with intestinal PCM infection mimicking stricturing CD and to perform a systematic review of the available cases reported on gastrointestinal PCM infection.
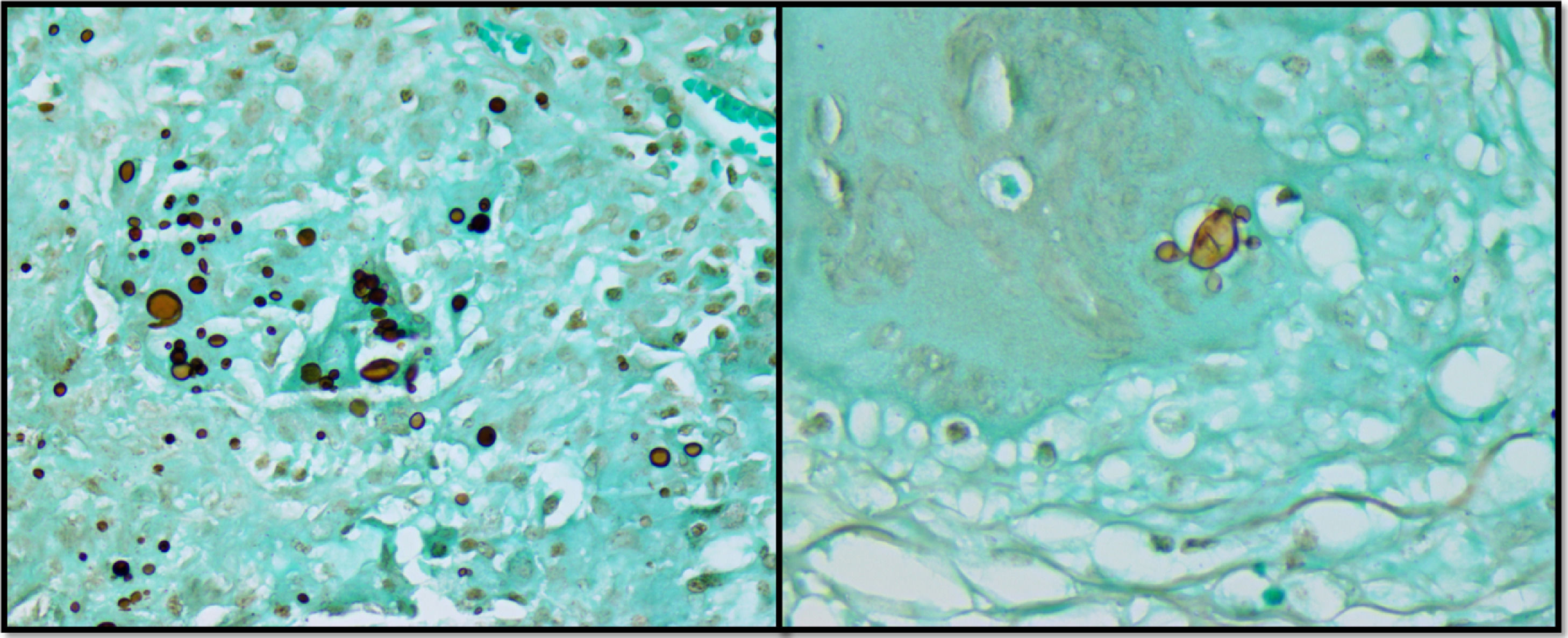
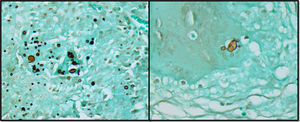
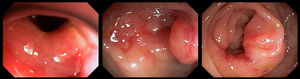
Case reportA previously healthy and asymptomatic 32-year-old female patient sought care in the emergency department presenting with acute pain in the lower right abdomen, associated with signs of peritoneal irritation and abdominal distension. The oral cavity and skin were unremarkable. A computed tomography scan was suggestive of appendicitis. The chest radiograph was normal. The patient underwent urgent surgery, which identified a severe suppurative perforated ileitis. A right colectomy with ileocolonic anastomosis was performed. The anatomopathological study showed chronic granulomatous inflammation of the ileum, large intestine, and pericolic lymph nodes, without evidence of inflammatory bowel disease. Fungal structures shaped as a ship's pilot wheel in Grocott-Gomori's staining were identified, suggestive of Paracoccidioides spp. ileitis and colitis (Figure 1).
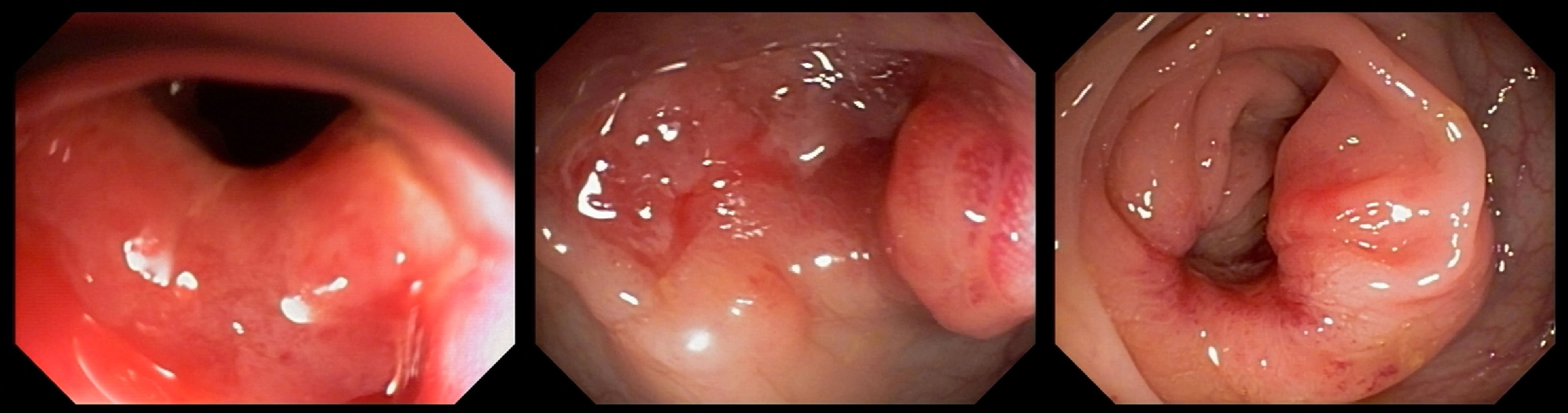
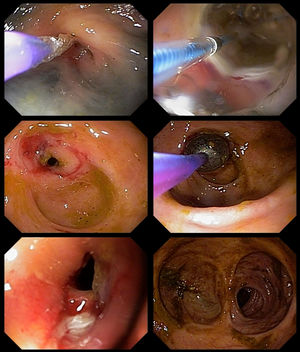
In the following two weeks, the patient presented vomiting, alternating diarrhea with constipation, and severe abdominal pain. An urgent colonoscopy was performed, which showed multiple colonic deep ulcers, which prevented the scope of reaching the ileo-colonic anastomosis (Figure 2). The anatomopathological study of biopsies was unspecific and staining for fungal structures was negative. At that time, she was started on amphotericin B deoxycholate 50 mg daily for 28 days according to her weight, with good improvement of symptoms. Afterward, she was kept on maintenance therapy with itraconazole 200 mg daily.
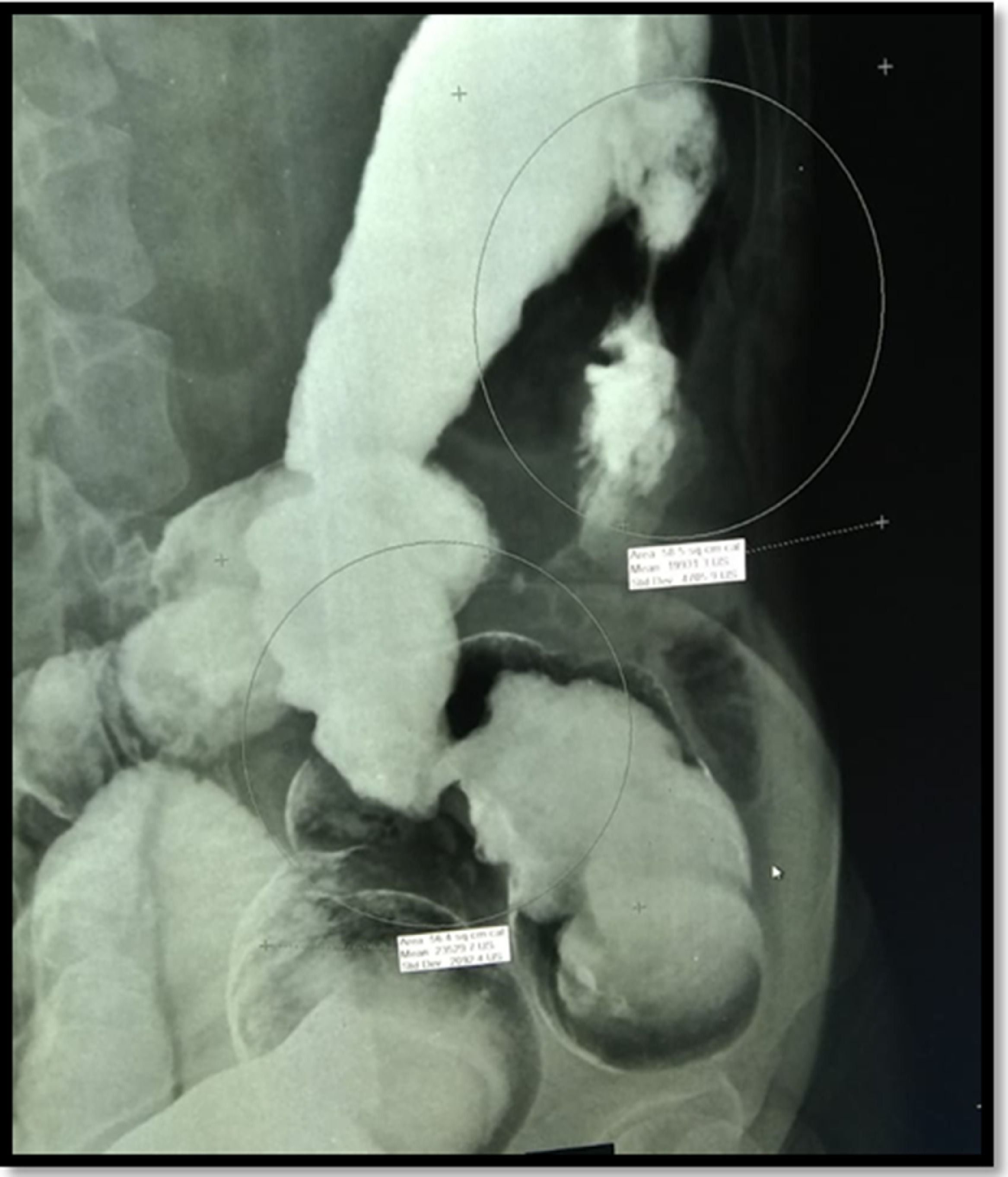
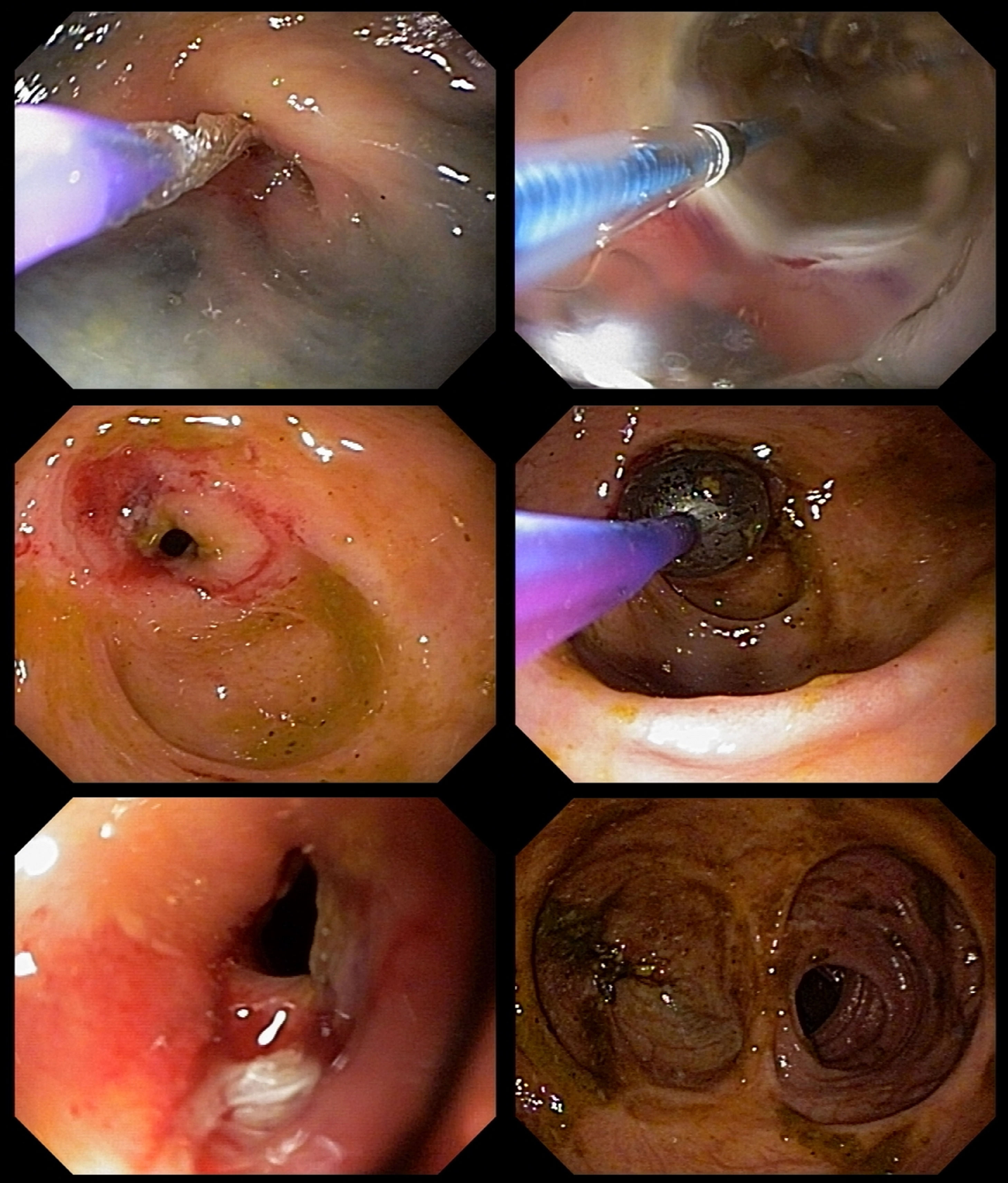
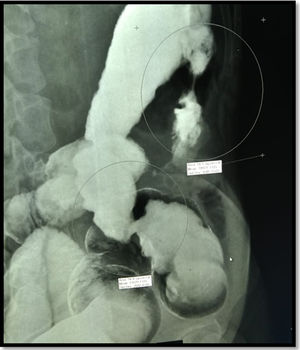
One month after ending the treatment with amphotericin B, a second colonoscopy was performed because of persistent abdominal pain, which showed a stricture in the left colon. A barium enema was performed, showing two short strictures (Figure 3). Endoscopic Balloon Dilation (EBD) was performed (Figure 4) with a mild short-lived improvement in symptoms. A few days after the second EBD, the patient presented to the emergency department with signs of bowel obstruction, requiring an emergency total colectomy. The anatomopathological study was unspecific and staining for fungal structures was negative.
After the surgery, symptoms of the patient were resolved. She is currently using maintenance therapy with itraconazole, with a plan of completing 18 months of treatment.
Materials and methodsThis study was carried out under the recommendations contained in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.9 Our systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO), maintained by York University (CRD42021246015).
Data sourcesStudies were retrieved using the terms described in Appendix A. Searches were run in November 2020 on the electronic databases Scopus, Web of Science, MEDLINE (PubMed), BIREME (Biblioteca Regional de Medicina), LILACS (Latin American and Caribbean Health Sciences Literature), SciELO (Scientific Electronic Library Online), Embase and Opengray.eu. References of included studies were screened for relevant records. There was no language or date of publication restrictions. The reference lists of the retrieved studies were submitted to manual search.
Inclusion criteria and outcomesCase report or case series studies were eligible for selection. If there was more than one study published using the same case, the most recent study was selected for analysis. Studies published only as abstracts were included, as long as the data available made data collection possible. Studies written in languages other than English, Spanish or Portuguese were excluded.
Study selection and data extractionAn initial screening of titles and abstracts was the first stage to select potentially relevant papers. The second step was the analysis of the full-length papers. Two independent reviewers extracted data using a standardized form after assessing and reaching consensus on eligible studies. The same reviewers separately assessed each study and extracted data about the characteristics of the subjects and the outcomes measured. A third reviewer was responsible for clearing divergences in study selection and data extraction
Quality assessmentMethodological quality assessment of case reports and case series was performed by two independent authors using the tool presented by Murad et al.10 Divergences were discussed with a third reviewer until consensus was reached. Since questions 5 and 6 of the original tool are mostly relevant to cases of adverse drug events, we modified them to better suit the cases of paracoccidioidomycosis. Therefore, we considered question 5 as ‘was there a response to the specific treatment for paracoccidioidomycosis?’ and question 6 as ‘was there a histological confirmation of the diagnosis?’.
Statistical analysisSimple descriptive statistics, such as the mean and Standard Deviation (SD), frequency, and median were used to characterize the data. Data were summarized using RStudio (version 4.0.2).
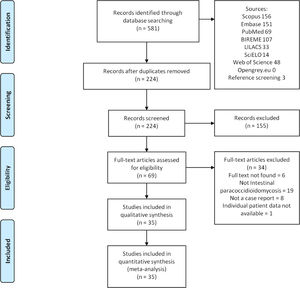
ResultsUsing the search strategy, 581 references were found and 224 references were excluded because they were duplicates. After analyzing titles and abstracts, 155 references were excluded and 69 full-text papers were analyzed. In the final analysis, 34 references were included, including 46 cases. A flowchart illustrating the search strategy is shown in Figure 5. Studies included were either a case report or a case series.
Brazil, Peru, Argentina, Colombia, and England included all cases (65.2%, 26%, 4.3%, 2.1%, and 2.1%, respectively). The baseline features are described in Table 1. A total of 46 patients were included, 32 (71.1%) males, mean age 35.2 years (range 5 to 68 years old). All patients were diagnosed with paracoccidioidomycosis (Paracoccidioides spp.). Inflammatory bowel disease was present in one case, with ulcerative colitis.
Baseline features in 46 patients with gastrointestinal paracoccidioidomycosis.
The most common clinical presentation was abdominal pain and weight loss, present in 31 (67.3%) cases, followed by diarrhea and cervical, axillary, inguinal, or other sites of lymphadenopathy (65.2% and 36.9%, respectively). Cutaneous manifestation and fever were present in 16 (34.7%) patients and lower gastrointestinal bleeding was present in 14 cases (30.4%). Only 7 (15.2%) patients presented with oral ulcers. In 10 (21.7%) cases, the disease presentation mimicked CD. The most common gastrointestinal locations were the large intestine, small intestine, and mouth (78.2%, 52.1%, 19.5%, respectively). In the intestine, the most specific locations were ileum, cecum, ascending, and transverse colon (20.5%, 19.1%, and 10.9%, respectively). Colonoscopy was performed in 28 (60.8%) patients and 45 (97.8%) underwent biopsy. The main characteristics of these and other tests are described in Table 2.
Summary of systematically reviewed clinical cases of gastrointestinal paracoccidioidomycosis.
| Reference | Country | Age | Sex | Clinical presentation | GI involvement | IBD | Crohn-like behavior | Treatment | Outcome | Quality assessment |
|---|---|---|---|---|---|---|---|---|---|---|
| Vieira11, 2001 | Brazil | 60 | M | Weight loss, coughing, dyspnea, dysphonia, ulcerated anal lesion, sphincteral hyponony | Large intestine, rectum | No | No | SFD | Recovery | High |
| Muñoz Urribarri12, 2006 | Peru | 5 | M | Vomiting, abdominal pain, fever | Large intestine | No | No | ICZ, SI | Recovery | High |
| Duani13, 2012 | Brazil | 24 | M | Fatigue, weight loss, diarrhea, hematochezia, abdominal pain, fever | Large intestine | No | Yes | SMZ-TMP, AmB, ICZ, SFD | Recovery | High |
| Penna14, 1979 | Brazil | 8 | F | Anorexia, diarrhea, hematochezia, abdominal pain, fever | Large intestine, rectum | No | Yes | SMZ-TMP | Recovery | High |
| Bravo15, 2010 | Peru | 39 | F | Weight loss, diarrhea, hematochezia, abdominal pain, fever | Large intestine | No | No | AmB | Death | Moderate |
| Benard16, 2013 | Brazil | 58 | F | Weight loss, diarrhea, fever | Large intestine | No | No | ICZ | Recovery | High |
| Benard16, 2013 | Brazil | 56 | F | Two colonic polyps found on routine colonoscopy | Large intestine | No | No | ICZ, endoscopic removal of the polyps | Recovery | Moderate |
| Alcántara17, 2017 | Peru | 29 | F | Weight loss, diarrhea, hematochezia, abdominal pain, fever, dizziness | Large intestine, rectum | No | No | AmB, ICZ | Recovery | Moderate |
| Alcántara17, 2017 | Peru | 24 | M | Fatigue, weight loss, diarrhea, dizziness, dyspnea | Small and large intestine | No | No | AmB, ICZ | Recovery | Moderate |
| Alcántara17, 2017 | Peru | 29 | M | Weight loss, diarrhea, abdominal pain, severe anemia | Large intestine | No | No | ICZ, SMZ-TMP | Recovery | Moderate |
| Martinez18, 2006 | Brazil | 22 | M | Weight loss, diarrhea, abdominal pain, fever | Small and large intestine | No | Yes | ICZ | Recovery | Moderate |
| Verona19, 1998 | Peru | 36 | M | Weight loss, abdominal pain, anal fissure, abdominal mass | Small and large intestine | No | No | ICZ | Recovery | Moderate |
| Bravo20, 2011 | Peru | 34 | M | Upper palate, nose, mouth and right foot ulcers, weight loss, diarrhea, hematochezia | Large intestine | No | No | AmB, ICZ | Recovery | Moderate |
| Hahn21, 2003 | Brazil | 22 | M | Weight loss, vomiting, abdominal pain, hepatomegaly, intestinal obstruction | Small intestine | No | No | SMZ-TMP, KCZ, AmB, FCZ, ICZ, SI | Recovery | High |
| Meyer22, 1982 | Brazil | 9 | F | Diarrhea, vomiting, abdominal pain, abdominal mass | Small and large intestine | No | No | SFD, SMZ-TMP, SI | Recovery | Low |
| Healy23, 2020 | England | 52 | M | Diarrhea, hematochezia, fever | Large intestine | UC | No | ICZ, SI, Posaconazol, Voriconazole, Azathioprine | Recovery | Low |
| Galeazzi24, 2011 | Brazil | 68 | F | Weight loss, diarrhea, abdominal mass | Small and large intestine | No | Yes | ICZ, SFD, AmB | NR | Moderate |
| Vinagre25, 2004 | Brazil | 22 | M | Weight loss, diarrhea, hematochezia, abdominal pain, fever | Large intestine | No | No | NR | NR | High |
| Freitas Junior26, 1991 | Brazil | 36 | M | Anorexia, vomiting, abdominal pain, fever | Small intestine | No | No | SI | Recovery | Moderate |
| Gava27, 2015 | Brazil | 20 | M | Weight loss, hematochezia | Mouth, small and large intestine, rectum | No | Yes | AmB | Recovery | High |
| Ribas28, 2008 | Brazil | 27 | M | Weight loss, diarrhea, hematochezia, vomiting, abdominal pain | Large intestine | No | No | SMZ-TMP, FCZ | Recovery | Moderate |
| Rocha29, 1997 | Brazil | 26 | M | Diarrhea, abdominal pain, ascites | Small and large intestine, rectum | No | No | KCZ, SI, Sulfisoxazole | Recovery | High |
| Rocha29, 1997 | Brazil | 30 | M | Anorexia, weight loss, diarrhea, abdominal pain | Large intestine | No | No | Antifungal therapy | Death | Moderate |
| Lomazi8, 2018 | Brazil | 13 | M | Weight loss, diarrhea, hematochezia, abdominal pain | Large intestine, rectum | No | Yes | ICZ, AmB | Recovery | Moderate |
| Chojniak30, 2000 | Brazil | 57 | NR | Weight loss, diarrhea, abdominal pain | Small and large intestine | No | No | KCZ, SI | Recovery | Moderate |
| Hossne31, 2005 | Brazil | 48 | M | Weight loss, abdominal pain | Small and large intestine | No | Yes | SI | Recovery | Moderate |
| Gorodner32, 2004 | Argentina | 59 | M | Weight loss, abdominal pain | Mouth, small intestine | No | No | SI | NR | Moderate |
| Rüssel33, 2016 | Argentina | 63 | M | Intestinal infarction, ischemia of the right lower limb | Small and large intestine | No | No | ICZ, SI | Death | Moderate |
| Golçalves34, 1996 | Brazil | 12 | F | Weight loss, diarrhea, hematochezia, vomiting, abdominal pain, fever, hepatomegaly, splenomegaly | Small and large intestine, rectum | No | No | SMZ-TMP, AmB | Recovery | High |
| Cury35, 2000 | Brazil | 43 | M | Weight loss, vomiting, abdominal pain | Small and large intestine | No | Yes | SMZ-TMP | Recovery | Moderate |
| Rodríguez36, 2018 | Colombia | 26 | M | Weight loss, diarrhea, abdominal pain | Small and large intestine | No | Yes | SMZ-TMP, AmB, SI | Recovery | Moderate |
| Troncon37, 1981 | Brazil | 23 | M | Vomiting, abdominal pain, fever, hepatomegaly, jaundice; ascites | NR | No | No | AmB, SFD | Recovery | High |
| Troncon37, 1981 | Brazil | 26 | M | Diarrhea, hepatomegaly, jaundice; ascites | NR | No | No | SFD | NR | High |
| Berni38, 2010 | Brazil | 53 | M | Weight loss, diarrhea, abdominal pain, fever, hepatomegaly | Mouth, large intestine | No | No | SMZ-TMP, AmB; ICZ; Pyrazinamide | Recovery | High |
| Berni38, 2010 | Brazil | 36 | F | Weight loss, abdominal pain, fever, hepatomegaly, jaundice; ascites | Mouth, large intestine | No | No | SMZ-TMP, AmB | Recovery | Moderate |
| Berni38, 2010 | Brazil | 50 | M | Weight loss, abdominal pain, fever, hepatomegaly, jaundice, oral lesions | Mouth, small intestine | No | No | SMZ-TMP, AmB | Recovery | High |
| Avritchir39, 1978 | Brazil | 23 | NR | Diarrhea, palpable abdominal mass | Small intestine | No | No | AmB | Recovery | Moderate |
| Avritchir39, 1978 | Brazil | 20 | NR | Diarrhea, abdominal pain, palpable abdominal mass | Small intestine | No | No | Antifungal therapy | Recovery | Moderate |
| Avritchir39, 1978 | Brazil | 36 | M | Diarrhea, hematochezia, abdominal pain, oral ulcers | Mouth, small intestine | No | No | NR | NR | Low |
| Fernández40, 1979 | Peru | 23 | M | Diarrhea, hematochezia, abdominal pain | Small and large intestine | No | Yes | NR | NR | Low |
| Fernández40, 1979 | Peru | 63 | M | Increased frequency of bowel movements | Large intestine | No | No | NR | NR | Moderate |
| Neves-Silva41, 2018 | Brazil | 36 | M | Weight loss, diarrhea, vomiting, oral ulcers, dysphagia | Mouth, small and large intestine, rectum | No | No | ICZ, SI | Recovery | High |
| Martinez42, 1984 | Brazil | 35 | F | Anorexia, fatigue, weight loss, diarrhea, hematochezia, vomiting, abdominal pain, fever, hepatomegaly, jaundice | Esophagus, small and large intestine | No | No | NR | Death | High |
| León43, 2010 | Peru | 66 | M | Weight loss, dyspnea; dry cough, oral lesions | Mouth | No | No | ICZ | Recovery | Moderate |
| León43, 2010 | Peru | 34 | M | Weight loss, diarrhea, oral ulcers, odynophagia, dry cough | Mouth, small and large intestine | No | No | ICZ, AmB | Recovery | Moderate |
| León43, 2010 | Peru | 40 | F | Weight loss, diarrhea, hepatomegaly | Large intestine | No | No | AmB | Death | Moderate |
AmB, Amphotericin B; F, Female; FCZ, Fluconazole; GI, gastrointestinal; IBD, Inflammatory bowel disease; ICZ, Itraconazole; KCZ, Ketoconazole; M, Male; MSZ, Mesalazine; NR, not reported; SFD, Sulfadiazine; SI, Surgical Intervention; SMZ-TMP, sulfamethoxazole-trimethoprim; UC, ulcerative colitis.
Medications used were described in 42 (91.3%) cases reported. Among these, 19 (41.3%) patients used itraconazole; 17 (36.9%) patients used amphotericin B; 12 (26%) underwent surgical intervention; 12 (26%) used sulfamethoxazole-trimethoprim and 6 (13%) used sulphadiazine. Considering only patients with available data, outcome was favorable for 34 (87.1%) patients; only 5 (12.8%) patients died. The mean time for signs and symptoms to disappear was 271 days, ranging from 18 to 2,555 days.
DiscussionThis was a systematic review of clinical presentations and outcomes of patients with PCM infection involving the gastrointestinal tract, mimicking inflammatory bowel disease in some cases.
PCM is an endemic mycotic disease found in Central and South America, typically causing asymptomatic pulmonary infection.44 The presented review found that most reported cases originated from South America, with a higher prevalence in Brazil (65.2%). The only reported case originating outside Central or South America was reported in England,23 but the patient had been to Costa Rica one year before symptoms from the infection began.
Patients with PCM infection are usually male, with a male/female ratio of 13:1 in Brazil,2 age average between 30 and 50 years old,44 and rarely occurring in children and teenagers.45 This infection predominates in males due to the protection provided by estrogen, which hinders the transformation of conidia and mycelial fragments into yeast cells, the pathogenic form of the fungus.6,46 Our analysis was compatible with the available epidemiological data, demonstrating a mean age of 35.2 years old and a higher prevalence in men (71.1%). Rural workers were the most frequently affected by PCM.1,2,6
The fungus is inhaled,45 which makes the lungs and the upper airways the first affected sites.47 The infection can evolve to two classic presentations: acute or subacute juvenile form, with important involvement of reticuloendothelial system, including spleen, liver, lymph nodes, and bone marrow dysfunction;2,45 or chronic adult form (90% of cases), with significant lung involvement and sometimes extrapulmonary lesions,47 including oral mucosa, skin, central nervous system, bowels, bone, adrenal, and genital organs. This represents endogenous reactivation years after initial contact.45 The fungus can disseminate to any part of the body by hematogenous or lymphatic route.15,47 Also, gastrointestinal involvement can happen due to ingestion of Paracoccidioides spp.15 In these cases, abdominal pain and diarrhea are the most common symptoms, also present in our patient. However, other manifestations were reported, such as hematochezia, mucus in stools, hepatomegaly, vomiting, and, less commonly, regurgitation, intestinal motility alterations, hiccups, and palpable abdominal mass.38
The gastrointestinal PCM can affect any part of the digestive system. In the presented review, the most common location of the disease was the colon, followed by the small intestine and mouth. Most intestinal alterations are found in the segments rich in lymphoid tissues, such as ileum, cecum, appendix, and the ascending colon.15,47 This information is compatible with our reported case, which demonstrated the presence of severe inflammation in the patient's ileum, large intestine, and pericolic lymph nodes, which first mimicked acute appendicitis. Afterwards, it presented with colonic ulcers which evolved into strictures, mimicking stricturing CD. Lesions in the esophagus, stomach, and anus are less common than in other sites.45
The most common symptoms found were abdominal pain (67.3%), weight loss (67.3%), and diarrhea (65.2%). This is also commonly found in patients diagnosed with inflammatory bowel disease.48 Intestinal PCM has been previously described as a condition that can mimic other diseases, especially CD,6 since this chronic auto-inflammatory condition can affect any part of the digestive tract.48 Therefore, intestinal PCM should be considered as a differential diagnosis for inflammatory bowel disease in endemic areas. In the presented systematic review, 10 patients developed a clinical syndrome that mimicked CD, presenting colonoscopies with multiple superficial or deep colon ulcers intercalated by areas with normal mucosa.13,14,18,35,36 This information highlights the importance of endoscopic tests and biopsies for adequate diagnosis of intestinal ulcers.
PCM infection may be opportunistic in patients with reduced cellular immunity either caused by another disease or by immunosuppressant treatment.49 The most commonly found correlations are cancer, HIV infection, and the use of immunosuppressive drugs.6,34 Concomitant IBD was observed in two patients diagnosed with PCM infection in the current review: a man with Ulcerative Colitis23 and a woman with Chron's disease.50 The patient was on immunosuppressive medications to treat the disease. According to the reported cases, the impaired immunity caused by the immunosuppressive therapy might have increased the risk for fungal infection.
Regarding laboratory data, anemia was the most common finding, with a mean hemoglobin of 8.8 g/dL and a mean hematocrit of 28.2%. Anemia is a common finding in patients with the acute/subacute form of PCM infection, generally associated with bone marrow involvement.4 Mucosal involvement resulting in gastrointestinal bleeding in the forms of hematochezia (30.4%) and melena (2.1%), can also contribute to the development of anemia.
The best tool for diagnosis of PCM infection is the identification of fungal elements suggestive of the infection in fresh examination of sputum, lesion scraps, lymph node, or biopsy of the affected organ.2,49 Typical histology shows inflammation signs and granulomas rich in epithelioid and giant cells containing variable amounts of fungal forms.15 Anatomopathological study of the biopsies of the reported case described fungal structures shaped as a ship's pilot wheel in Grocott-Gomori's staining, usually found in this disease. In the current review, 97.8% of the patients underwent biopsy of the affected organ to confirm the diagnosis.
Paracoccidioides spp. are sensitive to most systemic antifungals. A Brazillian guideline49 published in 2017 is the current consensus for the clinical management of this disease. The guideline suggests the use of itraconazole 200 mg daily for 9 to 18 months in mild and moderate cases of PCM.49 For patients with severe and systemic forms of PCM, the use of amphotericin B in deoxycholate or lipid formulation is suggested for the time necessary for the patient's clinical stability and transition to oral medication.49 The antifungal therapy to the reported case was chosen based on this guideline. Furthermore, surgical intervention and antifungal therapy can be associated to achieve better results. Our review demonstrated that the cure rate of the infection was 87.1% and the most common treatment option was itraconazole (41.3%) followed by amphotericin B (36.9%). Sulfamethoxazole-trimethoprim and sulfadiazine are no longer used. The treatment goal is to reduce the fungal load and allow cellular immunity to recover, so the patient can live without disease relapses, achieving balance between parasite and host.49
After the establishment of the best treatment option, patients diagnosed with PCM infection must keep an outpatient follow-up in order to reach the cure criteria, which is based on clinical, mycological, radiological and immunological parameters.46,49 Clinical cure is achieved when the signs and symptoms of the disease are no longer present,46 which includes wound healing, regression of adenomegaly and stabilization of body weight.49 Micological cure represents negative results on mycological tests after efficacious treatment.46,49 Radiological criteria is based on the image investigation of the lungs, the most commonly affected organ by PCM.46,49 Immunological cure consists on the decrease of serum fungus antibodies, which become undetectable or at very low concentration.46,49 The term definitive cure must not be used, based on the impossibility of eradicating the fungus from the organism completely.49
According to our findings, surgical intervention was necessary in 15% of the cases. In these cases, surgery was not considered as a treatment for the PCM, but was used as therapy for PCM complications, such as intestinal obstruction21 or structuring disease;26,31 acute abdomen;26,32,41 exploratory laparotomy for diagnostic purposes;26,41 and management of complications due to a major comorbidity.33 In our reported case, the patient underwent two surgeries. The first was due to a suspicion of acute appendicitis and the second was an emergency total colectomy due to bowel obstruction.
In conclusion, PCM should be suspected in patients who live or recently visited endemic areas, such as Central or South America, specially as a differential diagnosis for inflammatory bowel disease. Symptoms mimicking inflammatory bowel disease, such as abdominal pain, weight loss, diarrhea, and blood in stools are also present in intestinal PCM. Histologic studies, such as biopsies or surgical resection areas, are necessary for adequate diagnosis. Antifungal drugs such as itraconazole for mild/moderate disease and amphotericin B for severe disease seem to be the first treatment option and achieve significant success rates. Nevertheless, the considerable mortality are to be pointed out, and these patients should be managed with celerity.
Scopus
TITLE-ABS-KEY ((“Paracoccidioidomycosis” OR “Paracoccidioides” OR “paracoccidioidal” OR “Blastomycosis” OR “Blastomyces”)) AND ((“colon” OR “intestinal” OR “bowel” OR “ileum” OR “colonic” OR “Colitis” OR “Inflammatory Bowel Disease” OR “Crohn”))
Web of Science
TOPIC: ((“Paracoccidioidomycosis” OR “Paracoccidioides” OR “paracoccidioidal” OR “Blastomycosis” OR “Blastomyces”)) AND TOPIC: ((“colon” OR “intestinal” OR “bowel” OR “ileum” OR “colonic” OR “Colitis” OR “Inflammatory Bowel Disease” OR “Crohn”))
Embase
(‘paracoccidioidomycosis’ OR ‘paracoccidioides’ OR ‘paracoccidioidal’ OR ‘blastomycosis’ OR ‘blastomyces’) AND (‘colon’ OR ‘intestinal’ OR ‘bowel’ OR ‘ileum’ OR ‘colonic’ OR ‘colitis’ OR ‘inflammatory bowel disease’ OR ‘crohn’)
PubMed
(‘Paracoccidioidomycosis” OR “Paracoccidioides” OR “paracoccidioidal” OR “Blastomycosis” OR “Blastomyces”) AND (“colon” OR “intestinal” OR “bowel” OR “ileum” OR “colonic” OR “Colitis” OR “Inflammatory Bowel Disease” OR “Crohn”)
SciELO
*("Paracoccidioidomycosis" OR "Paracoccidioides" OR "paracoccidioidal" OR "Blastomycosis" OR "Blastomyces") AND ("colon" OR "intestinal" OR "bowel" OR "ileum" OR "colonic" OR "Colitis" OR "Inflammatory Bowel Disease" OR "Crohn")
BIREME
(("Paracoccidioidomycosis" OR "Paracoccidioides" OR "paracoccidioidal" OR "Blastomycosis" OR "Blastomyces")) AND (("colon" OR "intestinal" OR "bowel" OR "ileum" OR "colonic" OR "Colitis" OR "Inflammatory Bowel Disease" OR "Crohn"))
LILACS
("Paracoccidioidomycosis" OR "Paracoccidioides" OR "paracoccidioidal" OR "Blastomycosis" OR "Blastomyces") [Palavras] and ("colon" OR "intestinal" OR "bowel" OR "ileum" OR "colonic" OR "Colitis" OR "Inflammatory Bowel Disease" OR "Crohn") [Palavras]
Opengrey.eu
("Paracoccidioidomycosis" OR "Paracoccidioides" OR "paracoccidioidal" OR "Blastomycosis" OR "Blastomyces") AND ("colon" OR "intestinal" OR "bowel" OR "ileum" OR "colonic" OR "Colitis" OR "Inflammatory Bowel Disease" OR "Crohn"
Quality assessment of included cases of gastrointestinal paracoccidioidomycosis.
| Reference | Case number | Selection | Ascertainment | Causality | Reporting | Quality assessment | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q 1 | Q 2 | Q 3 | Q 4 | Q 5 | Q 6 | Q 7 | Q 8 | |||
| Vieira11, 2001 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High | |
| Muñoz Urribarri12, 2006 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | High | |
| Duani13, 2012 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High | |
| Penna14, 1979 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High | |
| Bravo15, 2010 | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Moderate | |
| Benard16, 2013 | 1 | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Moderate |
| Benard16, 2013 | 2 | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Moderate |
| Alcántara17, 2017 | 1 | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | High |
| Alcántara17, 2017 | 2 | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Moderate |
| Alcántara17, 2017 | 3 | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Moderate |
| Martinez18, 2006 | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Moderate | |
| Verona19, 1998 | No | Yes | No | Yes | No | Yes | No | Yes | Moderate | |
| Bravo20, 2011 | Yes | Yes | No | Yes | Yes | Yes | No | Yes | Moderate | |
| Hahn21, 2003 | Yes | Yes | No | Yes | No | Yes | Yes | Yes | Moderate | |
| Meyer22, 1982 | No | Yes | No | Yes | No | Yes | Yes | Yes | Moderate | |
| Healy23, 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High | |
| Galeazzi24, 2011 | No | Yes | No | Yes | No | Yes | No | No | Low | |
| Vinagre25, 2004 | No | Yes | No | No | No | Yes | No | No | Low | |
| Freitas Junior26, 1991 | No | Yes | No | Yes | No | Yes | No | Yes | Moderate | |
| Gava27, 2015 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High | |
| Ribas28, 2008 | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Moderate | |
| Rocha29, 1997 | 1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Rocha29, 1997 | 2 | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Moderate |
| Lomazi8, 2018 | No | Yes | No | Yes | Yes | Yes | Yes | No | Moderate | |
| Chojniak30, 2000 | Yes | Yes | No | Yes | No | Yes | Yes | No | Moderate | |
| Hossne31, 2005 | No | Yes | No | Yes | No | Yes | Yes | Yes | Moderate | |
| Gorodner32, 2004 | No | Yes | No | Yes | No | Yes | Yes | No | Moderate | |
| Rüssel33, 2016 | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Moderate | |
| Golçalves34, 1996 | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High | |
| Cury35, 2000 | No | Yes | No | Yes | Yes | Yes | No | No | Moderate | |
| Rodríguez36, 2018 | No | Yes | No | Yes | Yes | Yes | Yes | Yes | Moderate | |
| Troncon37, 1981 | 1 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | High |
| Troncon37, 1981 | 2 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | High |
| Berni38, 2010 | 1 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Berni38, 2010 | 2 | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Moderate |
| Berni38, 2010 | 3 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | High |
| Avritchir39, 1978 | 1 | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Moderate |
| Avritchir39, 1978 | 2 | Yes | Yes | No | No | Yes | Yes | Yes | No | Moderate |
| Avritchir39, 1978 | 3 | Yes | Yes | No | No | No | No | No | No | Low |
| Fernández40, 1979 | 1 | No | Yes | No | Yes | No | Yes | No | No | Low |
| Fernández40, 1979 | 2 | No | Yes | No | Yes | No | Yes | Yes | No | Moderate |
| Neves-Silva41, 2018 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | High | |
| Martinez42, 1984 | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | High | |
| León43, 2010 | 1 | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Moderate |
| León43, 2010 | 2 | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Moderate |
| León43, 2010 | 3 | Yes | Yes | Yes | No | No | No | Yes | No | Moderate |
Q 1: Did the patient(s) represent the whole case(s) of the medical centre? Q 2: Was the exposure adequately ascertained? Q 3: Was the outcome adequately ascertained? Q 4: Were other alternative causes that may explain the observation ruled out? Q 5: Was there a response to the specific treatment for paracoccidioidomycosis? Q 6: Was there a histological confirmation of the diagnosis?; Q 7: Was follow-up long enough for outcomes to occur? Q 8: Is the case(s) described with sufficient details to allow other investigators to replicate the research or to allow practitioners make inferences related to their own practice?