The HIV-1 initial viral infection may present diverse clinical and laboratory course and lead to rapid, intermediate, or long-term progression. Among the group of non-progressors, the elite controllers are those who control the infection most effectively, in the absence of antiretroviral therapy (ART). In this paper, the TH1, TH2 and TH17 cytokines profiles are described, as well as clinical and laboratory aspects of an HIV-infected patient with undetectable viral load without antiretroviral therapy. Production of IL-6, IL-10, TNF-α, IFN-γ, and IL-17 was detected; in contrast IL-4 was identified. Host-related factors could help explain such a level of infection control, namely the differentiated modulation of the cellular immune response and a non-polarized cytokine response of the TH1 and TH2 profiles.
According to the World Health Organization, the number of people living with HIV was estimated at 36.7 million in 2016.1 In Brazil, from 1980 to June 2016, 842,710 cases of AIDS were reported and the country has annually registered an average of 41,100 cases, with the Central-West region accounting for 15.1%.2
Clinical course and laboratory parameters are distinct in each HIV-infected individual. According to the clinical progression to disease, patients can be divided into fast progressors, slow progressors, and long-term non-progressors (LTNP). The LTNP group comprises the HIV controllers with viral loads lower than 2000copies/mL,3 whereas the elite controllers present undetectable levels of plasma viral load and low viral replication in vivo, evidencing the most effective control of the infection with no antiretroviral therapy (ART).4
In LTNP an immune response seems to occur with specific CD4 and CD8 T cells that control disease progression, in particular with highly active HIV-specific cytotoxic T lymphocyte (CTL). In these individuals, CD4 T cells are not depleted and actively contribute to antiviral immunity with interferon gamma production and other antiviral chemokines.5
Considering the small number of LTNP patients and the rare opportunity to observe modulation of the HIV-specific response, this study sought to analyze the cytokine pattern by measuring TH1, TH2, and TH17 cytokines for a better understanding of CD4 and CD8 T cells modulation, as well as other immunological factors involved in viral control.
The study was approved by the Ethics Committee on Research of the Federal University of Mato Grosso do Sul under protocol number 1.431.913.
A 43-year-old female patient initiated medical follow-up in August 2003 at the Basic Health Unit of the Municipality of Ponta Porã, state of Mato Grosso do Sul – Brazil, and continued until 2017. After signing the Informed Consent, two 5-mL samples of peripheral blood were collected by venipuncture without anticoagulant. After separating the serum, the material was stored at −80° C for quantification of TH1 (IL-2, TNF, IFN-γ), TH2 (IL4, IL-6 and IL-10), and TH17 (IL-17) cytokines, using the BD Cytometric Bead Array (CBA) Human TH1/TH2/TH17 Cytokine Kit, according to the manufacturer's instructions, and the BD FACSCanto II flow cytometer. For the analysis of results, the FCAP array software was used. Data from medical records were also collected.
Besides HIV infection without ARV, the patient presented some comorbidities such as systemic arterial hypertension, obesity, diabetes mellitus type II, and dyslipidemia. During all years of HIV infection, the patient presented no signs clinical of immunodeficiency. Her recurrent complaints were of gynecological origin, such as alterations in menstrual flow, leucorrhoea and vaginal pruritus, in addition to complaints related to intermittent joint pain. Patient reported no smoking history or alcohol consumption. Because of referred eating-related and anxiety disorders, she was under the supervision of a nutritionist.
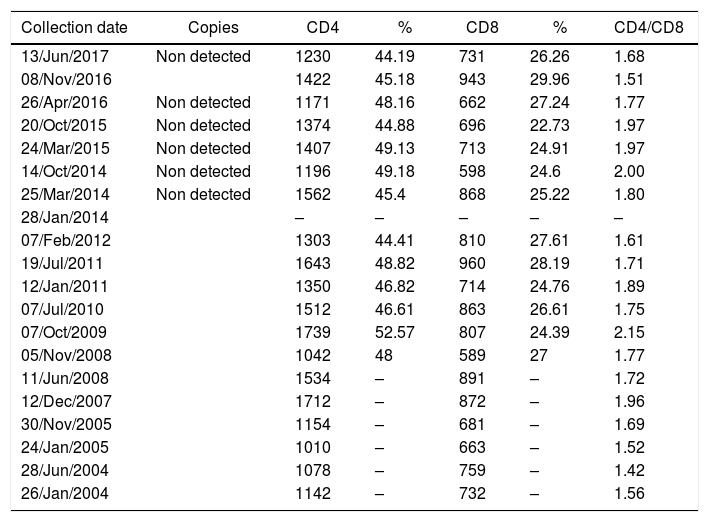
Laboratory data confirmed HIV infection, dyslipidemia, type II diabetes mellitus, and occasional changes in the urine test. No opportunistic diseases were diagnosed. From January 2004 to June 2017 HIV viral load was undetectable, and the average CD4 count was 1360cells/mm3, without considerable fluctuations (Table 1).
Viral load, CD4 and CD8 T cells count of a long-term non-progressor HIV infected patient.
| Collection date | Copies | CD4 | % | CD8 | % | CD4/CD8 |
|---|---|---|---|---|---|---|
| 13/Jun/2017 | Non detected | 1230 | 44.19 | 731 | 26.26 | 1.68 |
| 08/Nov/2016 | 1422 | 45.18 | 943 | 29.96 | 1.51 | |
| 26/Apr/2016 | Non detected | 1171 | 48.16 | 662 | 27.24 | 1.77 |
| 20/Oct/2015 | Non detected | 1374 | 44.88 | 696 | 22.73 | 1.97 |
| 24/Mar/2015 | Non detected | 1407 | 49.13 | 713 | 24.91 | 1.97 |
| 14/Oct/2014 | Non detected | 1196 | 49.18 | 598 | 24.6 | 2.00 |
| 25/Mar/2014 | Non detected | 1562 | 45.4 | 868 | 25.22 | 1.80 |
| 28/Jan/2014 | – | – | – | – | – | |
| 07/Feb/2012 | 1303 | 44.41 | 810 | 27.61 | 1.61 | |
| 19/Jul/2011 | 1643 | 48.82 | 960 | 28.19 | 1.71 | |
| 12/Jan/2011 | 1350 | 46.82 | 714 | 24.76 | 1.89 | |
| 07/Jul/2010 | 1512 | 46.61 | 863 | 26.61 | 1.75 | |
| 07/Oct/2009 | 1739 | 52.57 | 807 | 24.39 | 2.15 | |
| 05/Nov/2008 | 1042 | 48 | 589 | 27 | 1.77 | |
| 11/Jun/2008 | 1534 | – | 891 | – | 1.72 | |
| 12/Dec/2007 | 1712 | – | 872 | – | 1.96 | |
| 30/Nov/2005 | 1154 | – | 681 | – | 1.69 | |
| 24/Jan/2005 | 1010 | – | 663 | – | 1.52 | |
| 28/Jun/2004 | 1078 | – | 759 | – | 1.42 | |
| 26/Jan/2004 | 1142 | – | 732 | – | 1.56 |
copies of HIV/mL of plasma.
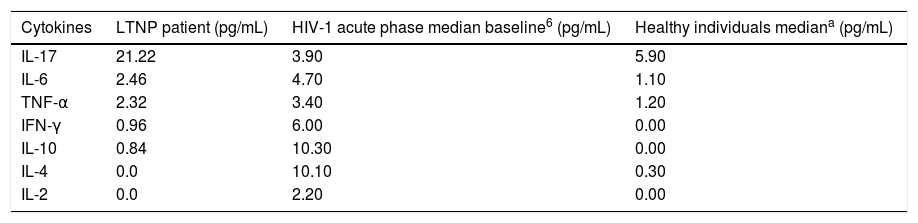
Patient's cytokine profile revealed the presence of IL-17, IL-6, TNF-α, IFN-γ and IL-10. No detectable concentrations of IL-4 or IL-2 were found (Table 2). The quantification of viral load, CD4 and CD8 T counts were not consistent with 13 years of HIV-infection, as viral load remained undetectable and CD4 counts had an average of 1360cells/mm3 throughout the follow-up period, with no major fluctuations. Thus, this patient was considered to be an elite controller.3
Cytokine profile in LTNP, HIV-1 in acute phase and healthy individuals.
| Cytokines | LTNP patient (pg/mL) | HIV-1 acute phase median baseline6 (pg/mL) | Healthy individuals mediana (pg/mL) |
|---|---|---|---|
| IL-17 | 21.22 | 3.90 | 5.90 |
| IL-6 | 2.46 | 4.70 | 1.10 |
| TNF-α | 2.32 | 3.40 | 1.20 |
| IFN-γ | 0.96 | 6.00 | 0.00 |
| IL-10 | 0.84 | 10.30 | 0.00 |
| IL-4 | 0.0 | 10.10 | 0.30 |
| IL-2 | 0.0 | 2.20 | 0.00 |
Note: Stacey et al.6
In HIV-1 infection, it is known that viral and/or host factors could regulate infection control. Among host-related factors, modulation of cellular immune response of LTNP patients are distinct from that of patients with rapid or intermediate progression.
HIV-1 spread through the lymphoid system occurs soon after infection, and the events during primary HIV-1 infection (PHI) determine the subsequent low level of viral replication.7 A significant difference in viral load was observed in LTNP PHI,8 indicating that patients capable of controlling HIV-1 infection emerge from the PHI with low levels of viral replication.7
When analyzed in vitro, CD4 T cells of LTNP in contact with HIV-1 present a significant population of HIV-specific memory cells. In addition, it has been demonstrated that CD4 T cells of elite controllers have highly avid antigen surface receptor for viral peptides such as Gag.9 These CD4 T lymphocytes are likely to have an indirect effect, stimulating a more effective CD8 T cell response in infection control.10
Another possibility is a direct antiviral effect of these HIV-specific cells with significantly low HIV-1 replication rate and vigorous CD4 T cell proliferation in response to HIV-1 Gag.7 CD4 T cells were identified as having a cytotoxic phenotype, capable of promoting lysis of autologous B cells coated with the cognate peptide.11 Appay et al.,12 have shown that HIV-1 infection is associated with an increase in circulating CD4 T cells containing perforins, even in PHI, expressing markers of cytotoxic activity in large amounts.
The classical function of CD8 T lymphocytes is cytotoxic activity in infected target cells. So, lysis of LTCD8-infected CD4 T cells would reduce viral replication and the amount of viral DNA. The cytotoxicity of these lymphocytes represents the main mechanism for the control of HIV infection. Thus, a modification in the modulation of CD4 T cells in the host's immune response is suggested, which would alter the profile of cytokines produced by the patient.13
Most HIV-infected individuals who progress to AIDS have an altered production of HIV-specific memory CD4 T cells and inability to produce IL-2 in response to HIV antigens.14 This dysfunction may be responsible for a subsequent decline in the cytotoxic response to the virus, allowing the progression to AIDS to occur.10
According to Imami et al.,15 when a detailed analysis of cytokine production in response to HIV antigens and peptides was performed, a mixed TH1 and TH2 response in patients who controlled viremia through a specific response to p24 viral antigen was observed in progressors and non-progressors, as well as in immunologically discordant progressors. The proliferation of CD4 T cells was accompanied by the initial production of IL-2 and IFN-γ, with a subsequent production of IL-4 and a peak production of IL-10, suggesting a cross-modulation between TH1 and TH2 cytokines. Such feature may be crucial for maintaining the cellular response to HIV-1 in non-progressors.15
IL-17 is produced by cells with a TH17 profile and plays an important role in enterocyte homeostasis in the gastrointestinal tract (GIT). IL-17 is also essential against bacterial and fungal infections at the site.16 A response of HIV-specific IL-17-producing CD4 T lymphocytes has been described and LTNP patients have higher IL-17 production when compared with progressors to immunodeficiency.17
The IL-17 production in the viral load of progressor and non-progressor patients was observed in a study where HIV-1 infected children with viral load lower than 50copies/mL presented significantly higher concentration of these cytokines. It was not possible to determine whether the decrease in IL-17 production is the cause or the effect of increased viremia.18,19
The patient presented a non-polarized cytokine production profile, since the production of IL-6, IL-10 (TH2), TNF-α and IFN-γ (TH1) was observed. IL-2 and IL-4 were not detectable, which could be due to the prolonged infection time of 13 years, as such cytokines are part of the characteristic pattern in the initial phase of infection. In addition, the production of IL-17 could be observed in a higher level, which corroborates the hypothesis of a greater production of IL-17 in LTNP patients with viremia lower than 50copies/mL. It is difficult to classify a patient soon after HIV infection or at the beginning of medical follow up, so this LTNP patient's data were evaluated after PHI, but the interference of other factors could not be evaluated. The cells that express perforin regardless of CD8 presence may have a role in controlling the infection. Thus, other studies evaluating HIV-infected patients in the PHI phase are necessary to clarify these mechanisms.
FundingStudent research grants were provided by the PIBIC-CNPq-UFMS and FUNDECT – CNPq Programs.
Conflicts of interestThe authors declare no conflicts of interest.