To evaluate the efficacy of the onabotulinum toxin type A in the treatment of HTLV-1 associated overactive bladder and its impact on quality of life (QoL).
MethodsCase series with 10 patients with overactive bladder refractory to conservative treatment with anticholinergic or physical therapy. They received 200Ui of onabotulinumtoxin type A intravesically and were evaluated by overactive bladder symptoms score (OABSS) and King's Health Questionnaire.
ResultsThe mean (SD) of the age was 52+14.5 years and 60% were female. All of them had confirmed detrusor overactivity on urodynamic study. Seven patients had HAM/TSP. The median and range of the OABSS was 13 (12–15) before therapy and decreased to 1.0 (0–12) on day 30 and to 03 (0–14) on day 90 (p<0.0001). There was a significant improvement in 8 of the 9 domains of the King's Health Questionnaire after the intervention. Hematuria, urinary retention and urinary infection were the complications observed in 3 out of 10 patients. The mean time to request retreatment was 465 days.
ConclusionOnabotulinum toxin type A intravesically reduced the OABSS with last long effect and improved the quality of life of HTLV-1 infected patients with severe overactive bladder.
The human T cell lymphotropic virus type 1 is the causal agent of the HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). About 10 million people are infected by HTLV-1 worldwide.1 HAM/TSP is characterized by back pain, hyperreflexia, spastic paraparesis, and Babinski sign.2 Manifestations of the genitourinary system such as erectile dysfunction, increased urinary frequency and urgency, with or without incontinence, and nocturia are documented in virtually all patients with HAM/TSP.3–5 Moreover, these manifestations may be detected in a large percentage of HTLV-1 subjects who do not fulfill criteria for HAM/TSP.5 The urinary complaints are responsible for serious impairment of quality of life, development of depression, and increased risk for upper urinary tract infection and kidney dysfunction due to increased intravesical pressure and residual urine volume.6–8 The main urodynamic findings in patients with urinary dysfunction associated with HTLV-1 are overactivity of the detrusor, sphincter-detrusor dyssynergia, and impaired bladder contractility.8,9 As only few studies have addressed the treatment of such events in this population, it remains undefined if the therapeutic interventions used in individuals not infected with HTLV-1 have the same response in those infected by the virus. The onabotulinumtoxin type A has been used with success to improve urinary symptoms in patients with overactive bladder symptoms due to multiple sclerosis or spinal cord injury.10,11 We had previously shown in a limited number of patients with urologic dysfunctions the short-term results of the use of onabotulinumtoxin.12 Here we extend this observation to a large number of patients, besides evaluating the long-term therapeutic response to onabotulinumtoxin type A in HTLV-1 infected patients with overactive bladder refractory to conservative treatment with anticholinergic drugs or physical therapy.
The aim of this study was to determine the effect of onabotulinumtoxin type A in controlling symptoms of lower urinary tract in patients infected with HTLV-1 refractory to conservative treatment with anticholinergic and pelvic floor physical therapy associated with parasacral or intracavitary neuromodulation (vaginal or anal).
MethodsPatients and case definitionsParticipants of the study were selected from a cohort study of 419 HTLV-1 infected subjects, of whom, 142 presented urinary symptoms. Eighty-six patients were on conservative treatment for HTLV-1 associated overactive bladder, 34 were not receiving regular treatment and 22 of these were considered refractory to drug therapy. Overactive bladder was defined according to International Continence Society (ICS) criteria13 and refractory overactive bladder was defined as failure to control urgency and incontinence using two different anticholinergic drugs in maximal tolerated dosage.14–16 All patients underwent an urodynamic study done before treatment.
The diagnosis of HAM/TSP and probable HAM/TSP was performed according to De Castro Costa criteria.17 Patients with probable HAM/TSP had urologic dysfunctions as the main symptoms. The amount of onabotulinumtoxin available was enough for only 10 patients and the first 10 cases who agreed to use the onabotulinumtoxin type A were enrolled in the study.
Administration of onabotulinumtoxin type APatients were anesthetized and positioned in lithotomy. All patients were on fluoroquinolone antibiotic prophylaxis. They received spinal or general anesthesia and 20mL of lidocaine gel into the urethra. Onabotulinumtoxin type A (Botox®, Allergan, Inc., Irvine, CA) was prepared according to the fabricant recommendation: A standard dose of 200 UI was reconstituted in 30mL of NaCl 0.9% solution. Then, the medication was injected in the detrusor muscle by cystoscopy in 30 different points of the supra trigonal region. One milliliter of the solution was administered in each site of application.18
The choice of 200UI dose was based in a previous study by Cruz et al. who showed that dose to have the same efficacy of 300UI in patients with urinary incontinence due to detrusor overactivity.
Clinical evaluationThe efficacy of the onabotulinumtoxin type A in controlling overactive bladder symptoms in HTLV-1 patients were assessed by a 3-day voiding diary and by the overactive bladder symptom score (OABSS). These parameters were assessed pre- and post-treatment. Moreover, patients were evaluated after 30, 90 and 365 days after the therapeutic intervention. The impact on quality of life was measured using the King's Health Questionnaire.19 We considered a high post void volume as over 50% of the estimated bladder capacity (400mL), as previously established by Asimakopoulos et al.20
Statistics analysisThe demographics and clinical data are described as mean±standard deviation (SD) or median (range). The Wilcoxon paired test was applied to compare pre- and post-intervention changes in frequency of voiding symptoms, OABSS and King's Health Questionnaire. p-Values<0.05 were considered statistically significant.
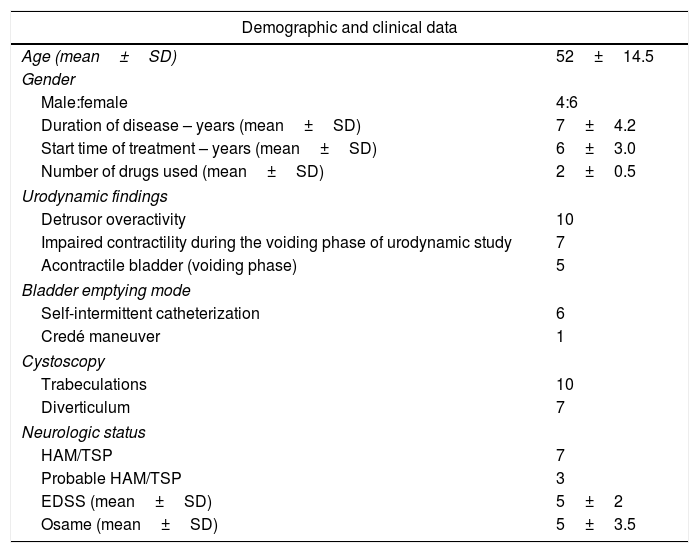
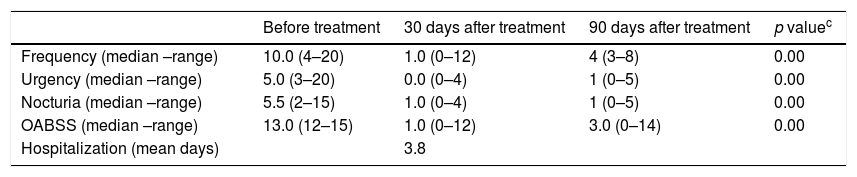
ResultsTable 1 summarizes demographic and clinical features of patients undergoing treatment with onabotulinumtoxin type A. All of them had already used at least two anticholinergic drugs (oxybutynin and propantheline bromide), given orally in full tolerated dosage. Of the 10 participants of the study, three had received in addition to oral, intravesical oxybutynin, but remained with urge incontinence. Two cases also had physical therapy with sacral, vaginal or trans-anal electrical stimulation with no improvement. The majority of the patients had illness duration for a long period. Detrusor overactivity was observed in all patients before therapy. Bladder empty dysfunction was detected in seven patients. Five of them had acontractile detrusor documented and six performed self-intermittent catheterization. The majority of these patients (n=04) had HAM/TSP, but in three cases urinary dysfunctions were the main neurologic symptoms. The impact of treatment on urological symptoms and OABSS after 30 days and 90 days of the therapeutic intervention is shown in Table 2. After application of onabotulinumtoxin type A, there was a significant reduction in the frequency (p=0.008), urgency (p=0.007) and nocturia (p=0.008). In addition, there was a significant reduction of OABSS measured 30, 90 and 365 days after the application of onabotulinumtoxin (p<0.005). No surgical complication was observed during the intraoperative period. Three out of 10 patients presented complications detected after therapy characterized by urinary tract infection and hematuria. Urinary retention were observed in two of these patients who were able to void spontaneously before treatment that persisted for 31 and 65 days after therapy. In all three cases, the infection was in the lower urinary tract and the patients responded promptly to ciprofloxacin. In patients who presented hematuria the symptoms disappeared within two days. Those patients who developed urinary retention needed to use self-intermittent catheterization. Despite being able to void spontaneously before therapy, these patients had high void residual volume.
Demographic, urodynamic and cystoscopic data of patients with refractory overactive bladder infected with HTLV-1 undergoing treatment with onabotulinumtoxin type A.
| Demographic and clinical data | |
|---|---|
| Age (mean±SD) | 52±14.5 |
| Gender | |
| Male:female | 4:6 |
| Duration of disease – years (mean±SD) | 7±4.2 |
| Start time of treatment – years (mean±SD) | 6±3.0 |
| Number of drugs used (mean±SD) | 2±0.5 |
| Urodynamic findings | |
| Detrusor overactivity | 10 |
| Impaired contractility during the voiding phase of urodynamic study | 7 |
| Acontractile bladder (voiding phase) | 5 |
| Bladder emptying mode | |
| Self-intermittent catheterization | 6 |
| Credé maneuver | 1 |
| Cystoscopy | |
| Trabeculations | 10 |
| Diverticulum | 7 |
| Neurologic status | |
| HAM/TSP | 7 |
| Probable HAM/TSP | 3 |
| EDSS (mean±SD) | 5±2 |
| Osame (mean±SD) | 5±3.5 |
| Before treatment | 30 days after treatment | 90 days after treatment | p valuec | |
|---|---|---|---|---|
| Frequency (median –range) | 10.0 (4–20) | 1.0 (0–12) | 4 (3–8) | 0.00 |
| Urgency (median –range) | 5.0 (3–20) | 0.0 (0–4) | 1 (0–5) | 0.00 |
| Nocturia (median –range) | 5.5 (2–15) | 1.0 (0–4) | 1 (0–5) | 0.00 |
| OABSS (median –range) | 13.0 (12–15) | 1.0 (0–12) | 3.0 (0–14) | 0.00 |
| Hospitalization (mean days) | 3.8 |
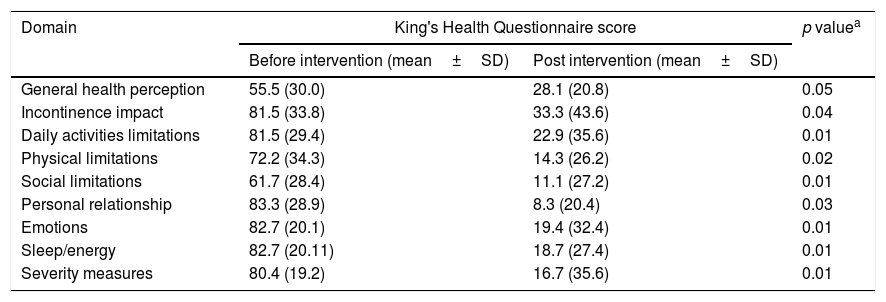
Table 3 shows the impact of the treatment on QoL. Of the nine domains comprised in the King's Health Questionnaire, a statistically significant reduction was demonstrated in eight of them. Regarding the general health perception, the statistical significance was not achieved but the p-value was 0.05.
Impact of onabotulinum toxin type A treatment in quality of life evaluated by King's Health Questionnaire.
| Domain | King's Health Questionnaire score | p valuea | |
|---|---|---|---|
| Before intervention (mean±SD) | Post intervention (mean±SD) | ||
| General health perception | 55.5 (30.0) | 28.1 (20.8) | 0.05 |
| Incontinence impact | 81.5 (33.8) | 33.3 (43.6) | 0.04 |
| Daily activities limitations | 81.5 (29.4) | 22.9 (35.6) | 0.01 |
| Physical limitations | 72.2 (34.3) | 14.3 (26.2) | 0.02 |
| Social limitations | 61.7 (28.4) | 11.1 (27.2) | 0.01 |
| Personal relationship | 83.3 (28.9) | 8.3 (20.4) | 0.03 |
| Emotions | 82.7 (20.1) | 19.4 (32.4) | 0.01 |
| Sleep/energy | 82.7 (20.11) | 18.7 (27.4) | 0.01 |
| Severity measures | 80.4 (19.2) | 16.7 (35.6) | 0.01 |
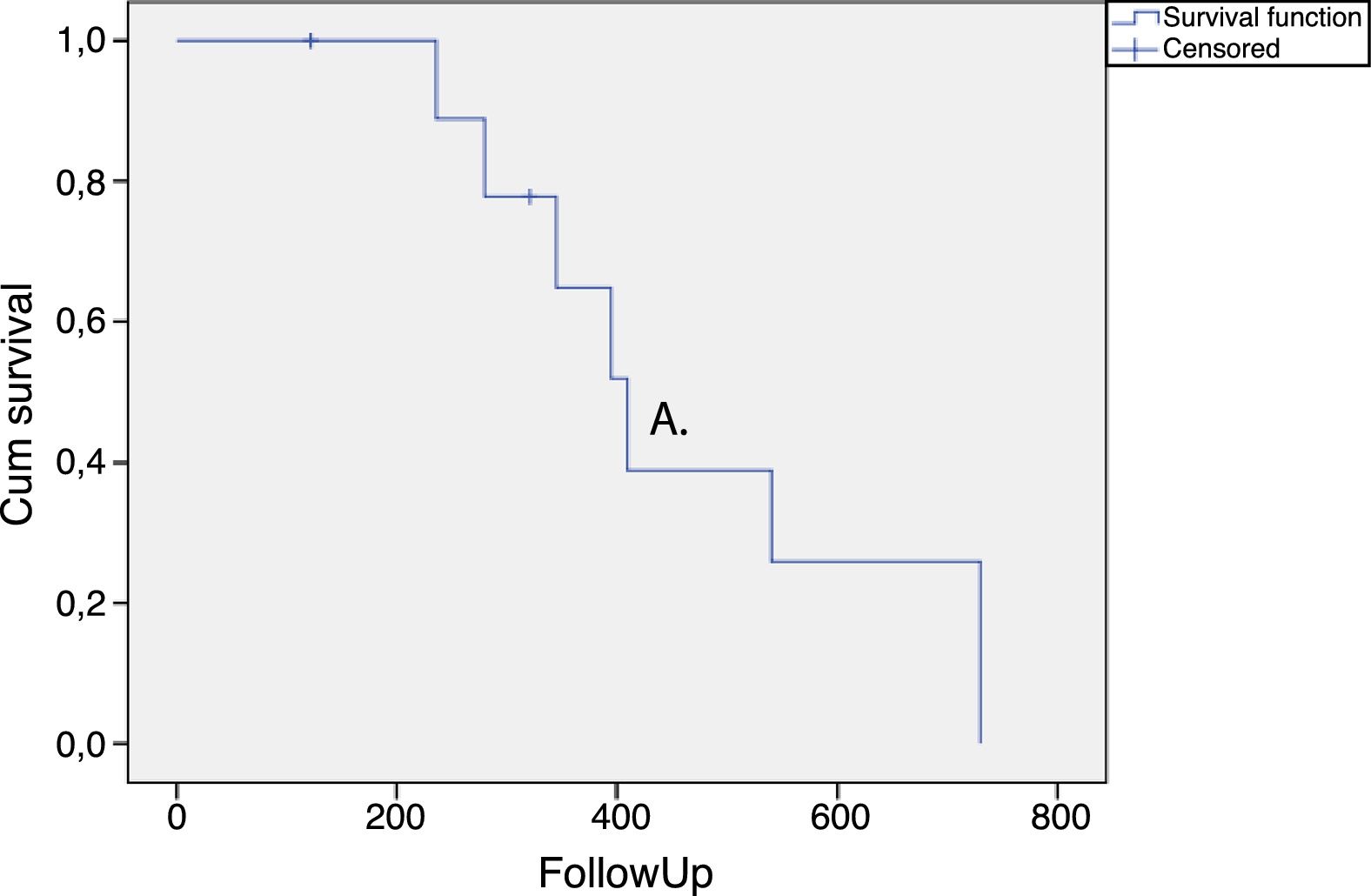
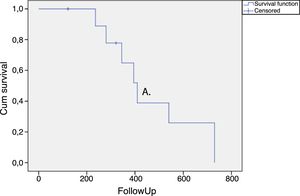
The duration of the treatment effect was assessed by survival analysis and expressed in a Kaplan–Meier curve (Fig. 1). The average time for requiring retreatment or returning to the previous treatment OABSS was 465.7 ± 66.3 days (Fig. 1).
DiscussionUrinary tract dysfunction (UD) plays an important role in the morbidity related to HTLV-1 infection.5,8,21,22 Initially, it was thought that urinary symptoms were caused by urinary tract infection, but one study failed to prove this hypothesis, and OAB symptoms are in fact due to neurological disease.8,23,24 Although several studies have investigated the physiopathology of UD, there are few studies assessing the efficacy of therapeutic strategies in HTLV-1 infected subjects. In this open label clinical study, we assessed the long-term therapeutic response of HTLV-1 infected subjects to onabotulinumtoxin type A. This drug was able to control the OAB symptoms, mainly urgency and incontinence for a long period (mean of 466 days) and improved QoL of the patients.
The cases enrolled in this study have experienced conservative treatment for a mean period of six years and had not achieved total control of the symptoms. In addition to the use of more than one drug orally, intravesical therapy had been applied in two patients. Although seven patients had impairment of detrusor contractility, we preferred to use a standard dose of the onabotulinum toxin type A in all patients (200UI), as recommended by Cruz et al.10
In the present study, OABSS was used to measure the OAB severity. This score evaluates the main symptoms of OAB giving different weights for each question related to the symptoms. Therefore, urgency and incontinence translate into more points in the score than frequency and nocturia. This score was also applied in a randomized controlled trial evaluating onabotulinumtoxin A in patients with multiple sclerosis.10 Our data show a significant decrease of OABSS in all periods of evaluation after the application of onabotulinumtoxin type A, which persisted for a long time (Fig. 1). The time for retreatment of the patients was higher than previously documented25 and several factors may explain this finding. It is known that the response to intravesical injections of onabotulinumtoxin type A is quite variable and dependent on the neurological disease related to urinary dysfunction.26 Moreover, we only offered retreatment when the patients requested it or when they presented the same or higher OABSS compared to the score observed before therapy.
UTI, hematuria and urinary retention were the most common complications, but their frequencies were similar to rates reported for patients with other neurologic diseases.10,27,28 As previous reported, UTI may occur after therapy.12,29 This fact may be explained by urine colonization in patients using self-intermittent catheterization due to high void residual volume or by endoscopic treatment. Anyway UTI is frequently documented in HTLV-1 infected patients. Regarding urinary retention, this complication was more frequent than the rate observed in other series.30 However, this was a predictable event, as all patients who presented urinary retention were not voiding normally, as they empty the bladder by involuntary contraction. In such cases, patients should be informed that urinary retention may be observed after therapy.
Gotoh et al. founded a direct relationship between OAB severity and impairment on quality of life (QoL). They also found that the symptoms with the highest bother score was frequency and urgency among patients under 50 years, urgency in the age range of 50–70 year, and incontinence in those over 80 years.31 In our study, incontinence was the symptom with the highest impact on QoL, but we did not investigate by age groups.
The limitations of the present study include the small sample size, the absence of a control group, and lack of continuity of care and the impossibility to apply all therapeutic armamentarium, such as posterior tibial stimulation and sacral neuromoduation. Also, we did not perform urodynamic study in the post-treatment evaluation for ethical reasons. First, there was no doubt about the clinical improvement and second the conduction of an urodynamic study by minimally invasive technique was not available in our service.
We recently showed that endocavitary (vaginal and anal) electrical stimulation combined with pelvic physiotherapy is an effective treatment of HTLV-1 associated urinary dysfunction.32 Here we found that onabotulinumtoxin type A promoted improvement in urinary symptoms and on QoL with acceptable rates of complications and may be used in patients infected with HTLV-1 with urinary incontinence.
ConclusionOnabotulinumtoxin type A should be considered in the treatment of overactive bladder associated to HTLV-1 refractory to anticholinergic drugs and physical therapy.
Conflicts of interestThe authors declare no conflicts of interest.
To Cristiano Franco for the assistance in manuscript translation and revision.
To Paulo Lessa and Maria Emilia Pedreira Freire de Carvalho Foundation.