Infection by human T-cell lymphotropic virus type 1 (HTLV-1) occurs in lymphocytes, which travel throughout the body, thus affecting several target organs and causing varied clinical outcomes, particularly in populations that are underserved and do not have access to healthcare. However, the mechanism of pathogenesis is not yet fully understood. The TAX and HTLV-1 basic leucine zipper factor (HBZ) proteins maintain viral persistence and affect pathogenesis through cell proliferation and immune and inflammatory responses that accompany each clinical manifestation. TAX expression leads to inhibition of transcription error control, OX40 overexpression, and cell proliferation in adult T-cell leukemia (ATL). OX40 levels are elevated in the central nervous system (CNS), and the expression of TAX in the CNS causes neuronal damage and loss of immune reactivity among patients with HTLV-1-associated myelopathy (HAM). HBZ reduces viral replication and suppresses the immune response. Its cell compartmentalization has been associated with the pathogenesis of HAM (cytoplasmic localization) and ATL (nuclear localization). TAX and HBZ seem to act antagonistically in immune responses, affecting the pathogenesis of HTLV-1 infection. The progression from HTLV-1 infection to disease is a consequence of HTLV-1 replication in CD4+ T and CD8+ T lymphocytes and the imbalance between proinflammatory and anti-inflammatory cytokines. The compartmentalization of HBZ suggests that this protein may be an additional tool for assessing immune and inflammatory responses, in addition to those already recognized as potential biomarkers associated with progression from infection to disease (including human leukocyte antigen (HLA), killer immunoglobulin-like receptors (KIR), interleukin (IL)-6, IL-10, IL-28, Fas, Fas ligand, interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and mannose-binding lectin).
Human T-cell lymphotropic virus type 1 (HTLV-1) was the first human retrovirus described in lymphocytes from a patient with cutaneous T-cell lymphoma [1,2], followed by the identification of human T-cell lymphotropic virus type 2 (HTLV-2) in a patient with hairy cell leukemia and/or hairy cell tricholeukemia [3]. HTLV-3 and HTLV-4 have also been described in isolated areas of forests in the Republic of Cameroon [4,5]; however, to date, they have not been found in other geographic areas or related to clinical manifestations [6,7]. There are at least six HTLV-1 molecular subtypes (a, b, c, d, e, f) [8–10] and four HTLV-2 molecular subtypes (a, b, c, d) [11–13].
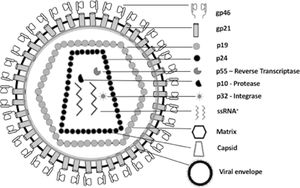
HTLV-1/2 are classified in the family Retroviridae and genus Deltaretrovirus. The viral particle is spherical and enveloped, measuring between 100 and 120 nm [14,15] (Fig. 1). The glycoproteins gp21 and gp46 are located in the viral envelope and are important for viral binding to the cell receptor and for envelope fusion with the cell membrane [11,16–19]. The protein capsid (formed by p15, p19, and p24) contains the viral genome, composed of two RNA molecules (single-stranded, positive polarity, and identical) [19-21], protease, reverse transcriptase (RT), integrase, and RNAse H, enzymes that facilitate viral replication [19,21,22]. RT is responsible for the transcription of single-stranded RNA into a double-stranded DNA molecule, which integrates into the genome of the host cell, becoming proviral DNA [19,21,23].
Schematic representation of the morphological components of HTLV-1/2. The nucleocapsid is composed of capsid proteins (p24 – genome protection), viral genomic RNA (ssRNA+ - genetic code), reverse transcriptase (p55 – RNA reverse transcription), protease (p10 - hydrolysis of viral peptides), and integrase (p32 – DNA proviral integration). The viral matrix is composed of the p19 protein and surrounds the nucleocapsid. The viral envelope is externally composed of a lipid bilayer plus the viral glycoproteins gp21 and gp46.
HTLV-1/2 share molecular and biological characteristics [11,21,24], and the integration of viral nucleic acids into the cellular genome establishes viral persistence and maintains and transmits the virus, which determines the various outcomes of infection [21].
HTLV-1/2 infect lymphocytes that are found in various body fluids, including blood, semen, vaginal secretions, and breast milk. The virus, which affects several target organs, is transmitted by blood and blood component transfusion, use of injectable drugs, organ transplantation, and unprotected sex [25–30]. There is therefore great variability in clinical manifestations associated with infection [31].
The mechanism of HTLV-1 pathogenesis is not yet fully understood. Among all of the regulatory proteins encoded by proviral DNA, the proteins TAX and HTLV-1 basic leucine zipper factor (HBZ) are essential for maintaining viral persistence and pathogenesis, possibly by inducing cell proliferation associated with the induction of immune responses [32].
HTLV-1 is a viral infectious agent with unique biological characteristics and diverse clinical manifestations. Because it still goes unnoticed in human populations, it is important to recognize the disease mechanisms previously associated with infection that result in the various known clinical manifestations.
The present review describes the main aspects of the immunopathogenesis of diseases associated with HTLV-1; it highlights the role of the viral proteins TAX and HBZ in the control of cell proliferation and activation of immune and inflammatory responses, and describes the multifactorial nature of diseases related to infection associated with the presence of immunogenic biomarkers in the host.
The role of HBZ and TAX in HTLV-1 infectionTwo HTLV-1 genes, TAX and HBZ, are extremely important in determining the infectivity of HTLV-1 and the leukemogenic process through regulation of the growth and survival of tumor cells [33–35]. TAX is an immunodominant HTLV-1 antigen with transformation and transactivation activities and is associated with dysregulation of immune responses in patients with HTLV-1-associated myelopathy (HAM), which leads to the main immunological changes observed in these patients [36–37]. TAX expression leads to persistent cell proliferation characterized by abnormal expansion of infected cells, generating DNA lesions characteristic of adult T-cell leukemia/lymphoma (ATLL) [38–41]. Inhibition of cell checkpoint activity to control transcription errors allows the proliferation of infected cells with damaged DNA [32].
TAX is involved in the overexpression of OX40, a member of the TNF receptor costimulation family capable of promoting proliferation and survival of effector and memory T cells and suppressing differentiation and activity of T regulatory cells (Tregs) [42]. Elevated levels of soluble OX40 have been detected in the central nervous system (CNS) of patients with HAM [43]. Expression of TAX in the CNS of patients with HAM can lead to direct or indirect neuronal damage through the loss of cells capable of activating and generating a specific immune response against TAX [44].
An association between a higher frequency of TAX-specific CD8+ T cells in the CNS of patients with HAM has been described, suggesting the active participation of these cells in the pathogenesis of the disease [45,46]. The presence of intrathecal antibodies against HTLV-1 has been associated with protective and pathogenic effects. Although the levels of intrathecal antibodies specific for HTLV-1 are inversely correlated with the proviral load, antibodies against TAX and Gag may cross-react with CNS tissues and lead to neurological damage [47–49].
Similar to TAX, HBZ is an immunogenic protein recognized by specific cytotoxic cell clones [50,51]. Expression of the HBZ protein induces a reduction in viral replication and suppression of immune responses [52–54]. HBZ is present in cells infected with HTLV-1, in both asymptomatic carriers and patients with HAM or ATL, and promotes the growth and survival of leukemic cells [55]. HBZ expression increases HTLV-1 infectivity, cell proliferation, and lymphoma [54,56]. Localization of HBZ in the nucleus suggests that HTLV-1 may increase viral persistence by reducing the translation of HBZ so that infected cells escape the immune response directed to this protein [57]. In turn, localization of HBZ in the cytoplasm of peripheral blood mononuclear cells (PBMCs) of patients with HAM occurs almost exclusively in CD4+ T cells and is independent of coexpression of CD25 [58]. These findings suggest that HBZ expression can be compartmentalized or co-occur with TAX expression, facilitating evasion of the virus from the host immune system and contributing to the pathogenesis of HAM.
The role of HBZ associated with its nuclear or cytoplasmic localization is related to the increased risk of developing HAM or ATLL. The intensity of the immune response and HBZ activation define the type of behavior of HTLV-1 infection [58]. The correlation between the intracellular compartmentation of the HBZ protein and the clinical outcomes of infection was reported, and it was proposed that the cytoplasmic presence of HBZ in leukocytes of patients with HAM is a biomarker of progression from infection to disease [39,58].
The available data suggest a crucial role of TAX and HBZ in the immune changes found in patients with HAM; however, the mechanism involved in the expression and regulation of these genes is still poorly understood. The most recent evidence indicates that TAX and HBZ exhibit antagonistic behaviors [59], with the action of HBZ being central in the pathogenesis of HTLV-1 infection; through its pleiotropic functions, HBZ initiates a viral strategy to increase the effectiveness of cell-to-cell transmission.
ImmunopathogenesisHTLV-1 infects different cell types (dendritic cells, macrophages, monocytes, CD8+ T lymphocytes) but mainly CD4+ T lymphocytes, which act as reservoirs for the virus [60]. In CD4+ T lymphocytes, HTLV-1 can remain latent for a long period [61] by maintaining a low rate of replication, which can cause genetic changes, induce cell proliferation, or even damage the CNS as the result of an inflammatory immune response [62–64].
Infection of CD4+ T and CD8+ T lymphocytes plays an important role in the immunopathogenesis of HAM [46,65] because it induces the production of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-1β, IL-12, and IL-6, which are involved in the mediation of inflammatory immune responses observed in infection [66,67,68]. Inflammatory chemokines, such as CXCL9 and CXCL-10, are also involved in the pathogenesis of HAM [69].
According to Hӧllsberg (1997), the pathophysiology of HAM is explained by three main mechanisms [70]: direct toxicity, autoimmunity, and surrounding damage. In the direct or cytotoxic toxicity theory, glial cells infected by HTLV-1 express surface viral antigens, and specific cytotoxic CD8+ T cells cross the blood-brain barrier to destroy infected glial cells through direct cytotoxic activity or the release of cytokines [71,72]. In turn, the autoimmunity theory suggests that a host antigen mimics an HTLV-1 antigen and triggers an autoimmune inflammatory process, resulting in neural injury [73,74]. The surrounding damage theory suggests that anti-HTLV-1-specific CD4+ T and CD8+ T lymphocytes migrate through the blood-brain barrier, reaching the CNS, where glial cells undergo cell damage because of the release of cytokines in response to lymphocytes infected by HTLV-1 [46,75–78].
TNF-α and INF-γ, which are secreted by CD4+ T lymphocytes (Th1 subpopulation), are the cytokines with the highest concentration in the cerebrospinal fluid (CSF) of patients with HAM [79]. Furthermore, other studies have shown that in addition to the predominance of Th1 cytokines (TNF-α, IFN-γ, IL-12), Th2 cytokines (IL-4, IL-10) are decreased in patients with neurological disease [66,80]. Patients with HAM show increased number of CD4+ T lymphocytes (Th1 subpopulation), with a higher proportion of IFN-γ- and TNF-α-producing cells than IL-10-producing cells [81]. This increase is also observed for CD8+ T lymphocytes that express the same cytokine pattern. A classification of disease activity in patients with HAM was proposed based on the concentration of CXCL10 and neopterin in the CSF. Patients with high levels of these cytokines have greater disease activity and appear to benefit most from the use of anti-inflammatory treatment [82–84]. Asymptomatic HTLV-1 carriers have an immunoregulatory mechanism characterized by increased IL-10 cytokine levels as a way to counteract the effects of TNF-α [65,81].
Asymptomatic patients with a high proviral load, as well as those with HAM, have higher IFN-γ expression than IL-10 expression [85,86], whereas asymptomatic patients with a low proviral load have similar levels of IFN-γ and IL-10 expression. This finding suggests that the imbalance between proinflammatory and anti-inflammatory cytokines is related to the development of HAM; that is, the pattern of the immune response in the host cell to HTLV-1 infection, combined with a high proviral load, may be important for the development of this severe neurological disease [65,87]. Recently, it was reported that the increased expression of adhesion molecules, such as CD49d, in T lymphocytes may contribute to the pathogenesis of HTLV-1-associated neurological disease in both asymptomatic and oligosymptomatic individuals [88].
Immunogenic profile of the hostThe interaction mechanisms of HTLV-1 with host responses and immunogenetic characteristics are important factors in the pathogenesis of HAM, ATLL, and other clinical manifestations associated with HTLV-1. It is still unknown why some individuals develop severe HAM or ATLL, others have moderate disease, and many others are asymptomatic. HTLV-1 is a genetically stable virus, and the same viral strain can generate various clinical outcomes. Numerous data on host genetic variations associated with immune responses to HTLV-1 infection, including human leukocyte antigen (HLA), killer immunoglobulin-like receptors (KIR), IL-6, IL-10, IL-28, Fas, Fas ligand, IFN-γ, TNF-α, and mannose-binding lectin, have been described as potential biomarkers associated with progression from infection to disease [89-91].
Recently, one study reported an association between polymorphisms in the TREX and SAMHD1 genes and increased proviral load of HTLV-1 among people with HAM [92,93]; similar results had also been described previously [88,94–96]. These data reinforce the need for further epidemiological genetic studies involving a larger number of people infected with HTLV-1 to better understand the effect of these markers on the pathogenesis and natural history of HAM.
There is also evidence of an association between genetic biomarkers and ATLL; that is, the genetic profile of the host can contribute to prognosis and can be an important additional tool in the management of affected individuals if properly implemented in endemic areas [97–101]. This finding demonstrates the importance of implementing these approaches in our environment.
HTLV-1 has a wide variety of interactions with the host and is associated with clinical manifestations that may involve the CNS, blood, eyes, skin, lungs, joints, intestine, bladder, thyroid, and heart, among other organs and systems [31]. The clinical complexity of the infection requires multidisciplinary care of the infected patient. Although the frequency of unfavorable clinical outcomes of HTLV-1 infections is considered low (5-10%), HTLV-1 infection may be associated with other clinical processes that need to be better defined. [102–104]. The increased frequency of reports of diseases associated with HTLV-2 [3,94,105–111] requires attention to rule out the participation of HTLV-2 in clinical outcomes, especially in areas endemic for this virus [112].
Summary and perspectivesHTLV-1 induces a persistent chronic infection. The development of associated diseases, such as HAM and ATLL, is multifactorial, involving factors related to the virus and to the immune and inflammatory responses of the host. The virus induces genetic changes in infected cells, cell proliferation, and even CNS injury from inflammatory immune responses. The genetic profile of the host is clearly associated with the balance between inflammatory and regulatory responses, predisposing or protecting against inflammatory diseases, such as HAM, caused by the virus. The development of ATLL is also related to the immunogenetic profile of individuals. Identification of prognostic markers in HTLV-1 infection is essential for predicting clinical outcomes and developing strategies for their prevention and management. In this sense, genome-wide association studies (GWAS) should be performed to screen possible new biomarkers.
A high HTLV-1 proviral load, HBZ, and some inflammatory cytokines are potential biomarkers for the development of diseases associated with HTLV-1.
We thank the Health Surveillance Secretariat from the Department of Diseases of Chronic Conditions and Sexually Transmitted Infections of the Ministry of Health of Brazil, the National Council for Scientific and Technological Development (CNPQ - Grant #301869/2017-0 ACRV; #312979/2018-5 RI; #304811/2017-3 MFRG), and the National Foundation for the Development of Private Higher Education – FUNADESP (http://www.funadesp.org.br/), grant #9600140 MFRG.