Vancomycin is widely prescribed to treat or prevent Gram-positive infections in pediatric liver transplant recipients. The objective of this prospective cohort study is to describe vancomycin pharmacokinetics and to evaluate the therapeutic target attainment after initial dose regimen.
Materials and methodsPatients with previous renal injury were excluded. Vancomycin therapy started with 40‒60 mg/kg/day. The pharmacokinetic parameters were assessed using two steady-state blood samples and the first-order kinetic equations. Therapeutic target was defined as vancomycin 24-hour Area Under the Curve/Minimum Inhibitory Concentration (AUC/MIC) ≥ 400 and < 600.
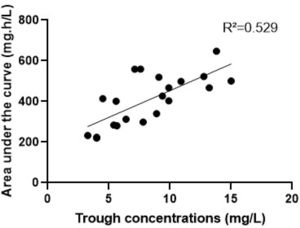
ResultsSixteen patients were included. The found vancomycin clearance, half-life, and volume of distribution were, respectively: 2.1 (1.3‒2.8) mL/kg/min, 3.3 (2.7‒4.4) hours, and 0.7 (0.5‒0.9) L/kg. With the initial dose, only 6 (37 %) patients reached the therapeutic target against Gram-positive pathogens with MIC 1 mg/L. After individual dose adjustments, all patients reached the target. The correlation between trough levels and AUC was low (R2 = 0.5).
ConclusionsPediatric patients with preserved renal function after liver transplantation have an increased volume of distribution for vancomycin, and most patients present subtherapeutic levels after the standard initial dosing regimen. With the vancomycin AUC-guided monitoring and dosing, it is possible to improve therapeutic target attainment.
Bacterial infections are among the main complications after liver transplantation in pediatric patients.1 However, these patients frequently receive the same empirical doses of broad-spectrum antibiotics as prescribed for other critically ill patients due to paucity of pharmacokinetic data.
Vancomycin is widely prescribed to treat or prevent Gram-positive infections in transplant recipients, especially due to the increasing risk of methicillin-resistant Staphylococcus aureus infections.2 Recent guidelines suggest dosing vancomycin based on Area Under the Curve/Minimum Inhibitory concentration (AUC/MIC) ratio of 400‒600 for optimal outcomes.3
Children naturally present an extensive variability of body composition, and sepsis-related inflammatory status, extensive fluid therapy and multiple organ dysfunction in critical health conditions affect drug disposition.4,5 Patients in general pediatric Intensive Care Unit (ICU) have a high incidence of subtherapeutic vancomycin levels or increased risk of nephrotoxicity after the standard initial dosage regimen.6
The aim of this study is to describe the pharmacokinetic parameters of vancomycin and therapeutic target attainment after the standard initial dosing regimen in pediatric liver transplant recipients.
We designed a prospective cohort study conducted in the pediatric ICU of a philanthropic hospital in São Paulo, Brazil between February 2020 and October 2022. Pediatric patients (< 18-years) in postoperative care of liver transplantation receiving vancomycin intravenously were included. Patients with renal impairment with an estimated creatinine clearance < 50 mL/min using the modified Schwartz formula7 or receiving renal replacement therapy were excluded. The study was approved by the local ethics committee. Guardians/Care takers of the children signed the informed written consent forms. Data collection included patient age, weight, height, primary diseases, date of surgical procedure, post-operative day ‒ defined as the period between the transplantation day and the first day of vancomycin monitoring ‒ concomitant drugs, graft weight to recipient weight, serum albumin, aspartate aminotransferase, alanine aminotransferase, urea, serum creatinine, gamma-glutamyl transferase, alkaline phosphatase, total bilirrubin, direct bilirubin, hemoglobin, hematocrit, platelet count, lactate, C-reative protein, white blood count, prothrombin time, partial thromboplastin time, and fibrinogen.
The vancomycin therapy started with empirical dose regimen of 10‒15 mg/kg every 6 hours with 1 hour infusion. For each patient, two steady-state blood samples were collected during the same dosing interval: a post distributional peak (1‒2 hours after the end of infusion) and a trough level (up to 1 hour before the next dose). Individualized dose adjustments were made to ensure target achievement of AUC/MIC ≥ 400 and < 600 throughout treatment or until patients were transferred to another hospital. The first-order pharmacokinetic analytic equations were used to estimate peak and trough concentrations, vancomycin clearance, elimination rate constant, biological half-life, and volume of distribution.8 We estimate vancomycin AUC using the logarithmic trapezoidal method, previously validated by Pai and Rodvold.8,9
Vancomycin concentrations were determined with the Cobas Integra 8000 analyzer (Roche Diagnostics, Switzerland) by fluorescence polarization immunoassay. The lower and upper limits of detection for this assay were 4 and 80 mg/L, respectively.
The GraphPad Prisma 7.0 software (GraphPad Software, San Diego, California) was used for data analysis. Categorical data are summarized as absolute frequencies (N) and percentages (%). Continuous demographic data, as well as the pharmacokinetics, are expressed as medians (interquartile range). The Pearson linear correlation was performed to assess the correlation between vancomycin 24-hour AUC and trough levels.
Five patients were excluded because they had renal impairment before receiving vancomycin. Sixteen patients with 48 measured vancomycin concentrations were included. The median patient age was 10 months. The main primary disease requiring liver transplantation was biliary atresia (81.2 %). All patients presented augmented renal clearance (creatinine clearance > 130 mL/min/1.73 m2).
Table 1 summarizes the characteristics of the patients at the beginning of therapeutic monitoring and the vancomycin pharmacokinetic parameters. After the vancomycin initial dosing regimen, only 6 (37 %) patients reached the therapeutic target against Gram-positive pathogens with MIC 1 mg/L; 9 patients (56 %) had subtherapeutic concentration (AUC < 400 mg.h/L); and 1 (6 %) patient had a supratherapeutic initial AUC value (> 600 mg.h/L). After individual dose adjustments, all patients reached the target. The median of the adjusted dose targeting AUC of 500 mg/L was 63 mg/kg/day. The relationship between trough levels and the 24-hour AUC is presented in Fig. 1 (R = 0.529). There were no reports of vancomycin-induced nephrotoxicity or infusion reactions during the period of study. No drug interactions were identified between vancomycin and concomitant drugs.
Clinical data and vancomycin pharmacokinetic parameters after the initial dosing regimen in pediatric post-liver transplant patients (n = 16).
IQR, Interquartile Range; Kel, Elimination Rate Constant; T1/2, Biological Half-Life; Clvan, Vancomycin Clearance; Vd, Volume of Distribution; MIC, Minimum Inhibitory Concentration.
Rankie et al., previously described the pharmacokinetics of vancomycin with data from 200 hospitalized children, and they found a median volume of distribution value between 0.47–0.61 L/kg.10 In our study, distribution volume values were slightly increased, ranging from 0.50–0.87 L/kg in transplant recipients, which justifies the high incidence of subtherapeutic levels after the initial dose. This occurs because, as vancomycin has hydrophilic properties, an increase in volume of distribution reflects a greater propensity for the drug to leave the plasma towards the extravascular compartments, meaning that a larger dose of a drug is needed to reach the required plasma concentration.11
The liver transplantation is an extensive procedure with substantial release of cytokines, so the systemic inflammatory state ‒ characterized by increased capillary permeability, interstitial edema, ascites, and pleural effusions ‒ can be responsible for the increases on volume of distribution leading to dilution in the extracellular space.12 Besides that, liver transplant recipients usually receive large amounts of fluid to maintain graft blood perfusion and systemic blood pressure. Kensuke Shoji et al. who also studied vancomycin pharmacokinetics in pediatric liver transplant recipients, also found higher values of volume of distribution, especially in the first few days after transplantation because of the systemic inflammatory and total-body fluid overload state.13
Impaired protein synthesis in the liver may also contribute to increases in the apparent volume of distribution, as hypoalbuminemia increases the fraction unbound of drugs, which can escape to the extravascular space. Although the vancomycin presents intermediate binding to albumin (∼55 %), this factor is relevant because even drugs with low protein binding, such as aminoglycosides (< 10 %), require higher doses in cases of severe hypoalbuminemia.14 The hyperbilirubinemia status may also decrease albumin binding and increase the volume of distribution, but with a lower intensity in drugs with intermediate protein bindings.12
The most common factors influencing vancomycin pharmacokinetics in previously published population pharmacokinetics studies are renal function descriptors.15 Since we have not evaluated patients with impaired renal function, we could not find a correlation between creatinine clearance and vancomycin clearance, and the found vancomycin clearance was comparable to the general children population. This is consistent with a previously published investigation in adult patients with augmented renal clearance, because drug clearance might reach saturation when renal function is enhanced.16 Cui-Yao He et al. evaluated a population pharmacokinetics of vancomycin in pediatric patients with augmented renal clearance and found a clearance value similar to ours – 2.3 mL/min/kg.17 Although acute kidney injury is a common complication after liver transplantation, more than half of critically ill children develop augmented renal clearance at the intensive care unit.18
Only approximately one third of the pediatric post-liver transplant patients reached the therapeutic target after vancomycin initial dosing regimen, meaning that the standard initial dosing regimen results in subtherapeutic vancomycin levels in most patients against pathogens with MIC 1 mg/L. Several studies have shown that initial dosing of 40 to 45 mg/kg/day was insufficient to achieve the AUC/MIC target in critically ill children. Pires et al. recommended a minimum vancomycin empirical dose of 60 mg/kg/day for pediatric patients in intensive care units with preserved renal function.6 Cui-Yao He et al. suggested that vancomycin dose should be increased to 75 mg/kg/day for infants and children with augmented renal clearance.17 In a large cohort study from Jennifer Le et al., doses of 90 to 100 mg/kg/day were necessary to achieve 90 % of the patients with AUC > 400 mg.h/L, but it resulted in excessive concentrations for lower MICs.19 In our cohort, an adjusted median dose of 63 mg/kg/day was optimal for a therapeutic target of 500 mg.h/L.
Ideally, the vancomycin therapeutic monitoring should begin within 24 to 48 hours of initial dosing.3 Even with similar characteristics and similar initial doses, our patients presented high interindividual variability of the vancomycin pharmacokinetic parameters and, consequently, high variability in serum concentrations. Additionally, vancomycin presented a narrow AUC range for optimal exposure, so the therapy needs to be individualized. A meta-analysis by Ye et al. suggested that appropriate therapeutic drug monitoring improve therapeutic target attainment of vancomycin in critically ill patients with unpredictable pharmacokinetics and decreases the incidence of drug-induced nephrotoxicity.20 The low correlation between trough levels and AUC shown in our study support the new recommendations to monitor vancomycin exposure through direct measurement of AUC to perform individual adjustments based on pharmacokinetic parameters.
The limitations of this study include the single center setting and small sample size. As most of the patients were aged < 2-years, caution should be used when extrapolating results to older patients. Additionally, it was not the purpose of this study to investigate the correlation of therapeutic target attainment with clinical or microbiologic outcomes, and in some cases, we were not able to follow the patient's entire clinical course because they were transferred to a different facility. Despite these limitations, our data contribute to a better understanding of drug disposition in this complex population.