Meningococcal disease by serogroup B has been a public health problem in Brazil in the last decades. The Brazilian Oswaldo Cruz Foundation has been working to develop a vaccine with detergent-treated outer membrane vesicles (OMV) and detoxified endotoxin (dLOS) from Neisseria meningitidis serogroup B prevalent strains. A phase I study, enrolling 26 adults (18–44 years of age) was performed using experimental vaccines combining B components and aluminum hydroxide as adjuvant. It was a dose escalation study testing vaccines made of 25, 50, and 100 µg OMV protein/mL (sum of both strains) and dLOS in half amount of total protein concentration, with three doses given two months apart. Adverse events were mild/moderate with frequency increasing with the amount of antigens. Pain in the site of injection was the most frequent reaction in all doses, reported in more than the 85% across vaccine groups. Considering all injections, cephalea was the most common systemic adverse event, detected in 11.1%, 17.2% and 32.1%, respectively with doses of 12.5 μg, 25 μg and 50 μg. High titers of total IgG (ELISA) were observed for the vaccine components before vaccination. Protective levels of bactericidal antibodies (titer ≥1:4) for both vaccine strains were also present. Considering a 4-fold increase of IgG titers compared to pre-immune values (seroconversion), 50%-70% of those who received intermediate and highest doses of antigens presented satisfactory response for OMV of N44/89 strain. The lowest dose vaccine induced no seroconversion for strain N44/89, and 11% for strain N603/95. For the three vaccines doses, 25% of seroconversion, in total IgG against LOS, was observed. Increased antibody bactericidal activity was observed for both strains in higher antigen concentrations. For IgG against LOS, all vaccine formulations showed 25% of seroconversion. In conclusion, MenB-Bio experimental vaccines were well tolerated and immunogenic, thus allowing phase II studies.
Meningococcal disease has been an important public health issue in Brazil, with endemic occurrence and recurrent epidemics and outbreaks since 1920.1–4 Serogroups B, C, Y and W of the Neisseria meningitidis have been the most frequently identified in meningococcal invasive disease in Brazil in the last two decades (http://www.saude.gov.br/ boletins-epidemiológicos). Following the introduction of the meningococcal C vaccine in the Brazilian basic schedule of immunization in 2010, the proportion of cases of serogroup C decreased from 40% to 27% in 2018, whereas serogroup B increased from 7% to 15%, with the caveat that the proportion of cases with missing serogroup remained approximately 50%.5–7 Serogroup B was initially the most important, with the phenotype, B:4,7:P1.19,15; L3,7,9 responsible for an epidemic in 1988 in São Paulo. This serosubtype still represented the predominant B phenotype from 2000 to 2011 and seems to remain the main strain nowadays.8,9
A universal meningococcal vaccine has been pursued over the years due to the severity and high mortality of these infections. For serogroups A, C, W and Y conjugated polysaccharide vaccines have been successfully used not only for the induction of protective bactericidal antibody titers but also for reducing the pathogens circulation by inducing herd immunity.10–12 For serogroup B, capsular polysaccharide-based vaccines have not been the strategy pursued because of the similarity of the polysaccharide structure to human cell sugars, which represents a potential risk for autoimmune response induction.13 Subcapsular antigens and, more recently, proteins with important roles in the onset of the disease, obtained by reverse vaccination, have been studied as new vaccine targets.14,15 Outer membrane vesicle-based vaccines (OMVs) have been evaluated in efficacy trials in Cuba, Brazil, New Zealand, and Norway. Efficacy has been demonstrated for adolescents and children aged more than four years. In a Brazilian study, protection against meningococcal disease caused by serogroup B was not elicited in younger children. The protection provided by OMV vaccines is associated with serum bactericidal antibodies which appear to be immunodependent on Por A surface protein. This characteristic is responsible for the specific serosubtype response induced by these vaccines.16–19
Following the epidemiological situation of meningococcal disease in Brazil since late 1990´s three Brazilian research institutions (Bio-Manguinhos/Fiocruz, Instituto Butantan and Instituto Adolfo Lutz) have collaborated to develop a group B meningococcal vaccine consisting of detergent treated OMV and detoxified endotoxin (dLOS) from Brazilian prevalent strains. From the early stages of development to the production process standardization, the two most prevalent meningococcal B serosubtypes in Brazil were B:4,7:P1.19,15:L1,3,7,9 (62%) and B:4,7:P1.7,1:L3,7 (9%), accounting for approximately 70% of all circulating serosubtypes. In order to develop a tailor-made vaccine, Bio-Manguinhos has developed a bivalent vaccine candidate (MenB-Bio) including OMV and dLOS from these prevalent strains, and dLOS with aluminum hydroxide as adjuvant. Good immunogenicity and protection against challenges in mice were demonstrated.20 The results supported initiation of Phase I clinical studies with MenB-Bio.21 The detoxified endotoxin was extracted from vaccine strain B:4,7:P1.19,15. Herein, we present the results of a phase I clinical study with this vaccine candidate to assess safety and to obtain preliminary information on immunogenicity of MenB-Bio.
Materials and methodsVaccine componentsThe bacteria growth and biomass separation were performed following Good Manufactoring Practice (GMP). Vaccine strains N44/89 (B:4,7:P1.19,15,L3,7,9) and N603/95 (B:4,7:P1.7.1,L3,7) were provided by Adolfo Lutz Institute, São Paulo, Brazil. The production process included cultivation of vaccine strains in 150 L bioreactor (B Braun Model Biostat UD 100) in Catlin MC.6 with 20 µM of Fe+3 and 42µM of Ethylenediamine-di – (O-hydroxy-phenylacetic acid) (EDDHA) to obtain outer membrane vesicles (OMV) and without EDDHA for lipooligosaccharide (LOS) production.22 In both processes bacteria growth was inactivated by heat (56°C/30 min) and the biomass separation was performed by continuous flow centrifugation (LAPX 4045GP-31G, Alfa Laval). The LOS depleted OMVs from vaccine strains were DOC-extracted from concentrate supernatant by tangential ultrafiltration in Centrasette stainless steel cassette holder with 0.9 m2 polyethersulphone Centrasette cassette and a molecular weight cutoff of 100 kDa (Pall Corporation). After the concentration step, OMV was treated with 2% (w/v) sodium deoxycholate (DOC) and harvested by ultracentrifugation (100,000 g for 2 h). The concentrate bulk was sterile-filtered using 0.22 µm Millipak®-200 with membrane polyvinylidene fluoride (PVDF- Millipore). The raw LOS was extracted from total biomass culture from N44/89 strain after hexadecyl trimethyl ammonium bromide (Cetavlon) treatment.23 The extracted LOS was further purified by gel filtration chromatography using 20 mM Tris-HCl, pH 8.5, containing 0.5% DOC and 5 mM Ethylenediaminetetraacetic acid (EDTA) as mobile phase on a single Sephacryl HR S-300 column (ID: 5.0X L: 100.0 cm). The LOS sample solution applied to the column was limited to 4% of bed volume and a LOS mass not higher than 600 mg. The purified LOS was detoxified (dLOS) with 0.2N NaOH in a water bath at 60°C/150 min.20
Experimental vaccinesThree formulations of the candidate vaccine used in the phase I study were prepared as follows. Vaccine 1: 25 µg of OMV protein (12.5 µg from N44/89 and 12.5 µg from N603/95) plus 12.5 µg of dLOS (N44/89) per mL; Vaccine 2: 50 µg of OMV protein (25 µg from N44/89 and 25 µg from N603/95) plus 25 µg of dLOS (N44/89) per mL; Vaccine 3: 100 µg of OMV protein (50 µg from N44/89 and 50 µg from N603/95) plus 50 µg of dLOS (N44/89) per mL. Those formulations had been tested in preclinical studies and were analogous to a commercially available vaccine. The vaccine candidates were lyophilized and reconstituted with diluent Al (OH)3 (2mg/mL) at the time of administration. Vaccine production and “in process” quality control were carried out in a GMP pilot plant production by the Quality Control Department from Bio-Manguinhos/Fiocruz.
Study design, safety assessment and monitoringVaccines were administered in three 0.5 mL intramuscular injections in the deltoid region at 8-week intervals (6–10 weeks acceptable). A sequential protocol was used in order to maximize safety for the volunteers. The first 10 volunteers received an injection containing 6.25 μg of each of the two strains of meningococcal B plus 6.25 μg of dLOS per dose, and were followed up for one month (group 1) after each dose to ascertain adverse events. Only after the first concentration was considered safe for human use after 30 days of follow-up, the second group of 10 volunteers was vaccinated with injections containing 12.5 μg of each of the two strains of meningococcal B plus 12.5 μg of dLOS (group 2). Likewise, only after 30 days of observation of this group disclosed no severe adverse events vaccination of the third group of 10 volunteers (25 μg of each of the two strains of meningococcal B plus 25 μg of dLOS - group 3) was started. After vaccination, study subjects stayed under observation at the Unit during two hours. Health professionals were trained for medical assistance in case of need, especially for anaphylactic reactions. Diary cards, telephone contacts, and visits to the trial unit were used to check for local and systemic adverse events. Diary cards were supplied to all volunteers so that they could record any adverse event for seven days after each injection. They were asked to bring the diary when they returned for clinical assessment on day 7. Another diary was then given for the period between day 7 and day 60. All subjects were given thermometers and oriented on how to measure and record the axillary temperature six and 12 h after vaccination and daily for seven days. Local and systemic adverse events (any sign or symptom) were evaluated in site by physical examination and interview on days 2 and 7 after each vaccine dose, and on day 30 after the last shot. Adverse events were also ascertained by telephone at days 1, 3, 15 and 30 after each immunization (except 30 days after the last shot when volunteers returned to the trial unit for their last study interview). Abnormal laboratory test results were evaluated by comparing baseline data collected prior to the first immunization to those obtained from blood samples taken 48 h after each shot. Blood samples were analyzed for full blood count, coagulation studies, platelet concentration, liver and renal function tests, and C-reactive protein. Auto-antibodies were tested at screening and 30 days after the last vaccine dose. The severity of adverse events was scored as 0 (absent); 1 (mild, easily tolerated); 2 (moderate discomfort, could interfere with some activities); 3 (intense, unable to perform usual activity), and 4 (life-threatening events). Fever was defined as axillary temperature ≥37.0°C. Hospital facilities were made available to manage severe adverse events. The study was approved by the Research Ethics Committee of the Instituto de Pesquisa Clínica Evandro Chagas (CAAE-0047.0.009.000-05) and was registered at the International Standard Randomized Controlled Trial Number (ISRCTN 75538667). An independent External Monitoring Committee, constituted by medical experts in clinical studies and meningococcal vaccines evaluated and followed up all study procedures to ensure the safety of participants and scientific integrity of the study.
Serum bactericidal assay (SBA)The strains N44/89 and N603/95 were used as the targets in SBA. Bacteria suspension from work seed lots prepared in Bio-Manguinhos, was streaked on a Columbia blood agar (Merck 1.10455.0500) with 5% horse blood (CBA) and incubated overnight at 37°C with 5% CO2. Afterwards, it was subcultured onto another CBA plate and incubated for 4 h at 37°C with 5% CO2. After 4 h growth the bacteria concentration was adjusted to 2 × 105 organisms/mL. Equal volumes (10 µL) of the bacterial suspension and human complement were added to 20 µL heat-inactivated test serum serially diluted twofold in bactericidal buffer in 96-well U-bottom microtiter plates (Greiner, Frickenhausen, Germany). The SBA were performed on serum samples from volunteers before immunization schedule (T0), before the second dose (T60) and one month after the third dose (T90) of immunization. Plasma selected from a suitable donor from Hemorio Hospital, Rio de Janeiro, Brazil) was used as the complement source at a 25% final concentration after 60 min of incubation. The number of CFU was determined at time zero and after 60 min of incubation, by allowing 10 µL of the reaction mixture to flow 8–10 cm, in lanes, down a CBA plate (the tilt method). After overnight incubation at 37°C with 5% CO2 colonies were counted. SBA titers were expressed as the reciprocal of the final serum dilution step giving 50% killing at 60 min compared to the number of CFU at time zero. The assay used three in-house serum controls, with low, media and high titers for each target strain.24,25
Enzyme-linked immunosorbent assay (ELISA)To detect anti-OMV total IgG, 96-well plates (ref. no. 3590; Corning-Costar, Corning, NY, USA) were coated with 100 μL/well of OMV (4 μg/mL) from N44/89 or N603/95 strains. Serum samples in two-fold serial dilutions in TBS with 0.05% Tween 20 (Merck, Schuchardt, Germany) and 5% FCS (Sigma-Aldrich, St. Louis, MO, USA) were incubated overnight at 4°C. The plates were incubated with anti-mouse IgG conjugated with alkaline phosphatase (whole molecule) (Sigma-Aldrich A-3688) diluted 1:2000. All sera were titrated in duplicate, and the titers were determined at an absorbance of 405 nm using a VERSA max tunable microplate reader (Molecular Devices, Sunnyvale, CA, USA). As the antibody standard, a positive post-vaccination serum was used in all experiments. The observed optical density was transformed to arbitrary units per milliliter by a sigmoidal standard curve (logit-log transformation) calculated from the values (1000 EU/mL) of the reference serum. To detect anti LOS total IgG, microtiter plates (Immulux ref 1000; Dynex Technologies, Chantilly, VA, USA) were coated overnight at room temperature with 100 µL/well of 10 µg/mL LOS-DOC micelles dissolved in PBS with 0.1% DOC. TBS with 5% bovine serum albumin (BSA) (Sigma-Aldrich ref A9418) was used as the blocking buffer for 2 h at 37°C. Sera samples were titrated in duplicate in TBS with 5% BSA and incubated for 3 h at 37°C. Conjugated antibodies (Sigma-Aldrich A-3688) were diluted 1:1000 and incubated for 2h at 37°C. The reaction was developed for 20 min with a phosphatase substrate (Sigma-Aldrich S0942). All sera were titrated in duplicate and titers were determined with absorbance at 405 nm in VERSA max tunable microplate reader – Molecular Devices. The total IgG antibody responses (EU/mL) against OMVs and LOS, were presented as geometric mean concentrations (GMC) of five serum samples (pool of five mice) by using a 4-parameter logistic curve-fitting analysis with SoftMaxPro software and documentation - Molecular Devices, against the standard curve generated by in-house serum with 1000 EU/mL of arbitrary units.26,27
Statistical analysis- •
Univariate analysis described the frequency distribution of sociodemographic characteristics of the participants.
- •
Safety. The frequency of local and systemic adverse events were reported by group, dose, severity, and timing of occurrence.
- •
Immunogenicity. The proportion of participants with SBA ≥8 and seroconversion (ELISA) to each meningococcal strain was calculated, by vaccine dose. Criteria for seroconversion were bactericidal titer <1/8 before vaccination and ≥1/8 after the third dose or ≥4 fold increase on bactericidal titers. The geometric mean of the reciprocal dilution (bactericidal titers) and Elisa units were estimated.
The study was a Phase I, unblinded, ICH/GCP compliant trial in 30 healthy adult volunteers aged 18-44 years, conducted from January 2006 to January 2007, at a special unit for immunobiological studies, which is an annex of an infectious disease hospital at the Fiocruz campus in Rio de Janeiro. Subjects were Fiocruz employees, not involved directly in the production of the vaccine. After reading, understanding and agreeing with an Informed Consent Form, potential volunteers were screened for their health status through their medical history, medical examination, routine biochemical and hematological tests, chest X-Rays and electrocardiogram. Before each dose of the vaccine, women were screened for pregnancy and HIV seropositivity, which was also an exclusion criterion. The study was conducted in accordance with the Declaration of Helsinki28 (World Medical Association Declaration of Helsinki 2001) and the European Clinical Trials Directive (European Clinical Trials Directive 2001)29 and the Brazilian legal and regulatory requirements (http://bvsms.saude.gov.br/1996/res0196_10_10_1996.html).30 The trial was approved by a research ethics committee (registration number CAAE-0047.0.009.000-05 at Institute of Infectology Evandro Chagas) and the Brazilian Regulatory Agency (Proc. ANVISA number: 25351.175427/05-24). The research protocol was registered at the International Standard Randomized Controlled Trial Number (ISRCTN no.: 75538667).
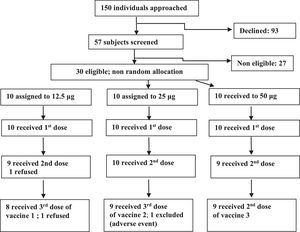
ResultsFrom January 2006 to January 2007, 150 individuals were contacted, 57 agreed to participate, 30 were eligible and were assigned to one of the three groups (Fig. 1). In the 12.5 μg group eight volunteers received all three doses, one received one dose and one received two doses. In the 25 μg group, nine volunteers received all three doses, and one received only two doses. In the 50 μg group nine volunteers received all three doses and one received only one dose. Twenty six volunteers were adherent to the protocol. Study subjects were mostly young adults in all vaccine groups. Distribution by sex was balanced in all but the 50 μg group, which included only men (Table 1).
There were no serious adverse events. Of the four volunteers who left the study before completing procedures, only one was due to an adverse event, in the 25 μg group, after the second dose, who presented local pain grade 3, local and thoracic exanthema, and moderate fever.
SafetyAll solicited local and systemic adverse events occurred in the first three days after vaccination and were of low or moderate intensity. Local pain in the site of injection was the most frequent reaction in all doses and vaccine groups. In the 50 μg group it occurred with similar frequency after all doses, whereas in the other vaccine groups the frequency decreased substantially in the second and third doses (Table 2). There was an increasing trend for local adverse events after larger doses, except for induration.
Frequency of local reactions according to dose and vaccine group.
| Vaccine group | Dose | Local pain | Erythema | Induration | Edema |
|---|---|---|---|---|---|
| 1st (n = 10) | 10 | 1 | 2 | 0 | |
| 12.5μg | 2nd (n = 9) | 7 | 0 | 1 | 0 |
| 3rd (n = 8) | 5 | 0 | 1 | 1 | |
| Total⁎ | 22/27 (81.5%) | 1/27 (3.7%) | 4/27 (14.8%) | 1/27 (3.7%) | |
| 1st (n = 10) | 10 | 0 | 0 | 0 | |
| 25μg | 2nd (n = 10) | 9 | 1 | 1 | 1 |
| 3rd (n = 9) | 6 | 0 | 2 | 1 | |
| Total⁎ | 25/29 (86.2%) | 1/29 (3.4%) | 3/29 (10.3%) | 2/29 (6.9%) | |
| 1st (n = 10) | 10 | 0 | 1 | 1 | |
| 50μg | 2nd (n = 9) | 9 | 2 | 0 | 2 |
| 3rd (n = 9) | 9 | 1 | 1 | 1 | |
| Total⁎ | 28/28 (100%) | 3/28 (10.7%) | 2/28 (7.1%) | 4/28 (14.3%) |
There was also an increasing trend for more intense adverse events after larger doses. Moderate or intense adverse events made up 13.6%, 17.3% and 30.3% of adverse events after 12.5 μg, 25 μg, and 50 μg doses, respectively (Table 4). Pooling together injections for each vaccine group, cephalea was the most common systemic adverse event, detected in 11.1%, 17.2% and 32.1%, respectively in groups 12.5 μg, 25 μg, and 50 μg. Axillary temperature ≥37.0°C was also frequent especially after the first dose of the 50 μg group (Table 3). Axillary temperatures ≥37.5°C occurred in 3.7%, 10.3% and 17.9% of all vaccinations, respectively in groups 12.5 μg, 25 μg and 50 μg (data not shown). Malaise and myalgia were also more frequent following the 50 μg vaccine (Table 4). The highest axillary temperatures were 37.5°C, 38.0°C and 38.5°C for the 12.5 μg, 25 μg and 50 μg groups, respectively. There was a trend for sooner occurrence and longer duration of adverse events with larger doses.
Frequency of systemic adverse events according to dose and vaccine group.
| Vaccine group | Adverse Event | Nausea | Malaise | Myalgia | Cephalea | Ax. Temp.≥37°C |
|---|---|---|---|---|---|---|
| 1st dose (n = 10) | 1 | 1 | 0 | 3 | 1 | |
| 12.5μg | 2nd dose (n = 9) | 1 | 1 | 0 | 0 | 4 |
| 3rd dose (n = 8) | 0 | 1 | 1 | 0 | 2 | |
| Total (%)⁎ | 7.4 | 11.1 | 3.7 | 11.1 | 26.0 | |
| 1st dose (n = 10) | 2 | 0 | 0 | 4 | 3 | |
| 25μg | 2nd dose (n = 10) | 1 | 1 | 1 | 1 | 3 |
| 3rd dose (n = 9) | 0 | 1 | 0 | 0 | 4 | |
| Total (%)⁎ | 10.3 | 6.9 | 3.4 | 17.2 | 34.5 | |
| 1st dose (n = 10) | 1 | 1 | 0 | 3 | 5 | |
| 50μg | 2nd dose (n = 9) | 0 | 2 | 1 | 5 | 2 |
| 3rd dose (n = 9) | 1 | 2 | 2 | 1 | 3 | |
| Total (%)⁎ | 7.1 | 17.9 | 10.7 | 32.1 | 35.7 |
Laboratory test abnormalities were apparent in hematological parameters, with increased number (compared to baseline data) of participants presenting levels below the reference values (Table 5). Those lower values were observed predominantly in women, even though they comprised 1/3 of the study subjects. In fact, among women the geometric mean of hemoglobin decreased 4–8% approximately, after the first dose in all three vaccine groups, whereas in men there was a 3% reduction in groups 12.5 μg and 50 μg and 0.7 increase in group 25 μg. A 15% increase in the geometric mean of leucocyte count was seen after the first dose of the 50 μg group whereas in the other vaccine groups it was less than 3%. There was also an increase in the percentage and number of neutrophils after all dose groups, but more evident in the 50 μg group, in which the increase in C-reactive protein was also more marked, consistent with an inflammatory response. One participant had laboratory test result consistent with auto-immunity (cardiolipin IgM of 20) after the third dose in the 50 μg group. No other clinically relevant laboratory test abnormality was detected.
ImmunogenicityAll participants had bacteridal antibody titers equal to or higher than 1:4 before and after vaccination (data not shown).
The three groups that received the experimental vaccines showed protective bactericidal antibody activity in pre-immune sera for both tested strains (Table 6). Conversely, seroconversion based on ELISA units against OMVs from N. meningitidis was more frequent for higher doses of N44/89 strains compared to the N603/95 strain (Table 7).
Geometric mean titers of bactericidal antibodies against N. meningitidis serogroup B strains N44/89 and N603/95 in serum samples, according to vaccine formulation.
Geometric mean of ELISA units against OMVs from N. meningitidis strains N44/89 and N603/95 in serum samples from vaccines, and proportion of seroconversion, according to vaccine formulation.
Total IgG antibody against OMVs from both strains presented high levels before vaccination (Table 7). For the intermediate and highest doses a fourfold increase was observed in 67% and 56% of vaccinated volunteers, respectively, for the N44/89 strain. Only the group that received a 12.5 µg dose did not show any seroconversion. The post-vaccination values for N603/95 strain were 11%, 34% and 34% for 12.5 µg; 25 µg and 50 µg/dose, respectively. Total IgG antibodies against LOS had a fourfold increase in 25% of the three vaccinated groups (Table 8).
Geometric mean of ELISA units against LOS from N. meningitidis strain N44/89 in serum samples from vacinated volunteers and proportion of seroconversion, according to vaccine formulation.
This was the first clinical trial of a new vaccine against serogroup B meningococcus totally produced in Brazil and based on the concept of a tailor-made vaccine, that is, a vaccine made based on the Brazilian epidemiological conditions. A dose-escalating design was used with the objective of finding the best balance between reactogenicity and immunogenicity.
The reactogenicity profile found in the current study was expected for this kind of vaccine and was similar to those obtained in other studies with higher frequency of adverse events after larger doses. Tappero et al. in 199931 found that the reactogenicity of the Cuban vaccine (50 μg) was higher than the Norwegian vaccine (25 μg). Studies comparing the Norwegian vaccine to the New Zealand vaccine, both with 25 μg OMV, have shown similar frequency of adverse events.22 A phase I/II study comparing two antigenic concentrations of the New Zealand vaccine, 25 μg and 50 μg/ dose, showed that the higher concentration induced more adverse events, without additional benefit in immunogenicity.32,33
In the current study, adverse events were mild or moderate, and no serious adverse event was observed. The most frequent local and systemic adverse events were local pain and low grade fever, respectively. In line with the studies mentioned above, we observed that adverse events increased with higher antigen concentrations, chiefly local adverse events. Similarly, as antigen concentration increased, there was a trend for sooner occurrence and longer duration of adverse events.
Clinical laboratory tests, in general, remained within normal limits, but reflected an inflammatory response expected for this kind of vaccine.
One caveat is the lack of an unvaccinated control group which would allow to distinguish systemic nonspecific adverse events that could have had other causes unrelated to the candidate vaccine. It is also important to consider that the sample size was not conceived to detect nor to compare less frequent adverse events, which will be addressed in phase II trials.
Immunological response was very good for strain B:4:P1.14.15 (N44/89) and moderate for strain B:4:P1.7.1 (N603/95), although the high level of antibodies before vaccination have impaired analysis of immunogenicity.
We used a very strict criterion for seroconversion, considering seroprotected individuals with bactericidal titers ≥1:8 or fourfold increase in titers. The most recent recommendations consider seroprotected individuals with ≥1:4 bactericidal titers or fourfold increase in titers.34–36 However, in the current study, adoption of this last recommendation would lead to the same results.
In summary, the Brazilian vaccine against meningococcus B has shown an acceptable safety profile and promising immunogenicity, which shall be further verified in a phase II study in seronegative children or with low level of antibodies before vaccination.