The diagnosis of congenital toxoplasmosis presents limitations and therefore new options are necessary. The analysis of amniotic fluid by real-time PCR has already proved effective for confirmation of fetal infection. However, its performance in other biological samples is not clear yet. The aim of this study is to better understand the role of real-time PCR in the blood of the mother and newborn as well as in the amniotic fluid and placenta in the diagnosis of congenital toxoplasmosis. This is a descriptive cohort study of pregnant women with toxoplasmosis followed up in Rio de Janeiro, Brazil. Real-time PCR was performed in samples of maternal blood, amniotic fluid, placenta, and blood of newborns. In addition, histopathological examination of placentas was performed, and data collected from babies were collected. 116 pregnant women were followed up and 298 samples were analyzed. One (0.9%) pregnant woman presented positive PCR in the blood, 3 (3.5%) in the amniotic fluid, 1 (2.3%) in the placenta and no newborn had positive PCR in the blood. Histopathological study was suggestive of toxoplasmosis infection in 24 (49%) placentas. Six (5.2%) newborns were diagnosed with congenital toxoplasmosis, and only cases with positive PCR in the amniotic fluid had correlation of the PCR result with the diagnosis of congenital infection. Both maternal and blood samples of newborns and placenta did not prove to be promising in the diagnosis of congenital toxoplasmosis. Further studies are needed to evaluate the real role of molecular diagnosis in other biological materials rather than the amniotic fluid.
Although it is considered a neglected infectious disease, toxoplasmosis affects one third of the world's population.1 In Brazil, its high prevalence suggests high exposure of the population to its infectious agent Toxoplasma gondii (T. gondii).2 When infection occurs during pregnancy it can lead to poor formation or even fetal loss. However, the vast majority of newborns are asymptomatic and may develop mainly ocular changes throughout life.3 Only 30% of infected pregnant women are symptomatic and even those asymptomatic may transmit the disease to the fetus.1 There is no consensus regarding the serological screening of toxoplasmosis during prenatal care.4 The treatment, when used during pregnancy, reduces both the possibility of transmission and the occurrence of severe sequelae in the baby.5 Screening of toxoplasmosis during prenatal care is done through serological tests and IgG avidity, which have limitations.6,7 Molecular tests allow greater agility and diagnostic accuracy.8 The high sensitivity and specificity of the Real-Time Polymerase Chain Reaction (RT-PCR) in the amniotic fluid led to the inclusion of this examination in the routine of care for pregnant women with toxoplasmosis in reference centers.9 Other biological samples such as blood, placenta, urine and cerebrospinal fluid have also been evaluated through molecular tests to determine their contribution to the diagnosis of congenital toxoplasmosis.8 However, the performance of these other biological specimens is still controversial.8
The aim of this study is to better understand the role of real-time PCR in the blood of the mother, the newborn as well as in the amniotic fluid and placenta for the diagnosis of congenital toxoplasmosis.
Material and methodsStudy designThis is a descriptive cohort study of pregnant women diagnosed with toxoplasmosis followed up at a referral center for congenital infections in Rio de Janeiro, Brazil, between June 2019 and December 2021. Pregnant women diagnosed with HIV infection, hepatitis B, C or using immunosuppressive therapy were excluded. Pregnant women were defined as a case of toxoplasmosis when presenting seroconversion of anti-T. gondiiantibodies of IgG class or presence of anti-T. gondii antibodies of both IgM and IgG class, by immunofluorometric assay – ELFA (VIDAS®, BioMérieux, France), associated with low or moderate IgG avidity or high IgG avidity if the test was performed with more than 16 weeks of gestation.
Real-time PCRSamples of peripheral blood, amniotic fluid and placenta were collected from pregnant women, and peripheral blood samples were collected from newborns. In all samples the Real-Time PCR (qPCR) test was performed using the TaqMan system using specific initiators to amplify the sequence of T. gondii of 529 pairs of bases. Genomic DNA was extracted from different sources using QIAamp® DNA Blood Mini Kit (QIAGEN, Netherlands) according to manufacturer's instructions. Nucleic acid concentration was estimated by UV spectrophotometry at 260 nm (Nanodrop 1000, ThermoFisher Scientific, USA). The detection of T. gondii by PCR was performed as already described.9,10 Approximately 10 ng of DNA were used in PCR with the primer mix (270F, 5′-AGAGACACCGGAATGCGATCT-3′ and 318R, 5′-TTCGTCCAAGCCTCCGACT-3′) and TaqMan probe (310T: 5′-FAM-TCGTGGTGATGGCGGAGAGAATTGA-TAMRA-3′) for the highly repetitive REP- 529 sequence. The reactions were performed in triplicate in a 7500 Fast Real-Time PCR (Applied Biosystems®) system at a final volume of 25 μL using TaqMan Universal Master Mix (ThermoFisher Scientific) PCR. Thermal cycling conditions included a 2 min to 50 °C stage for optimal AmpliErase UNG activity and denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s 60 °C for 1 min. The standard curves were established with serial dilution of 10 times of DNA of T. gondii (106 to 1 parasite/mL). The PCR efficiency was higher than 92% and the correlation coefficient was ≥0.99 in all tests. Samples with CT > 38 was classified as undetectable. Human RNaseP amplification was used as control to determine DNA quality and presence of PCR inhibitors, and it was performed with 20 × RNaseP Primer Probe (Applied Biosystems). The positive control consisted of a small amount (1‒10 copies/mL) of genomic DNA of the parasite.
The newborns were screened for congenital infection by means of the following tests: trans fontanelle ultrasonography, fundus oculi examination and serology for toxoplasmosis with identification of specific anti-T.gondii antibodies IgM and IgG class gondii. The criterion for the diagnosis of congenital toxoplasmosis in newborns was a positive PCR in amniotic fluid and/or presence of changes in screening tests at birth.11 When available, placentas were also referred for histopathological analysis.
Statistics and data analysisData were analysis with Epiinfo version 7.2.4.0. Exploratory data analysis was done with the description of categorical variables as absolute and relative frequencies and numerical variables as means.
The study was approved by the Research Ethics Committee of the National Institute of Women's, Children's and Adolescent Health Fernandes Figueira ‒ Fiocruz (CAAE: 14832713.7.0000.5268/Opinion Number: 3.587.173). All pregnant women signed the Free and Informed Consent Form.
ResultsA total of 116 pregnant women diagnosed with toxoplasmosis with a mean age of 25.8 years (minimum 14 and maximum 43) were studied. The majority (97/83.6%) were referred to the reference center by the public health service, followed by the private network (16/13.8%) and 3 (2.6%) sought care on spontaneous demand. Sixty (51.7%) women lived in the city of Rio de Janeiro and 56 (48.3%) in neighboring municipalities. Fifty-two (44.8%) pregnant women had not completed primary education and only 18 (15.5%) attended higher education.
Symptoms suggestive of acute acquired toxoplasmosis (fever, swollen lymph node, headache, sore throat, and myalgia) were reported by 14 (12.1%) women, while 102 (87.9%) were asymptomatic. Treatment for congenital infection was not prescribed in the units of origin for 29 (25%) pregnant women and in 15 (12.9%), incorrectly prescribed.
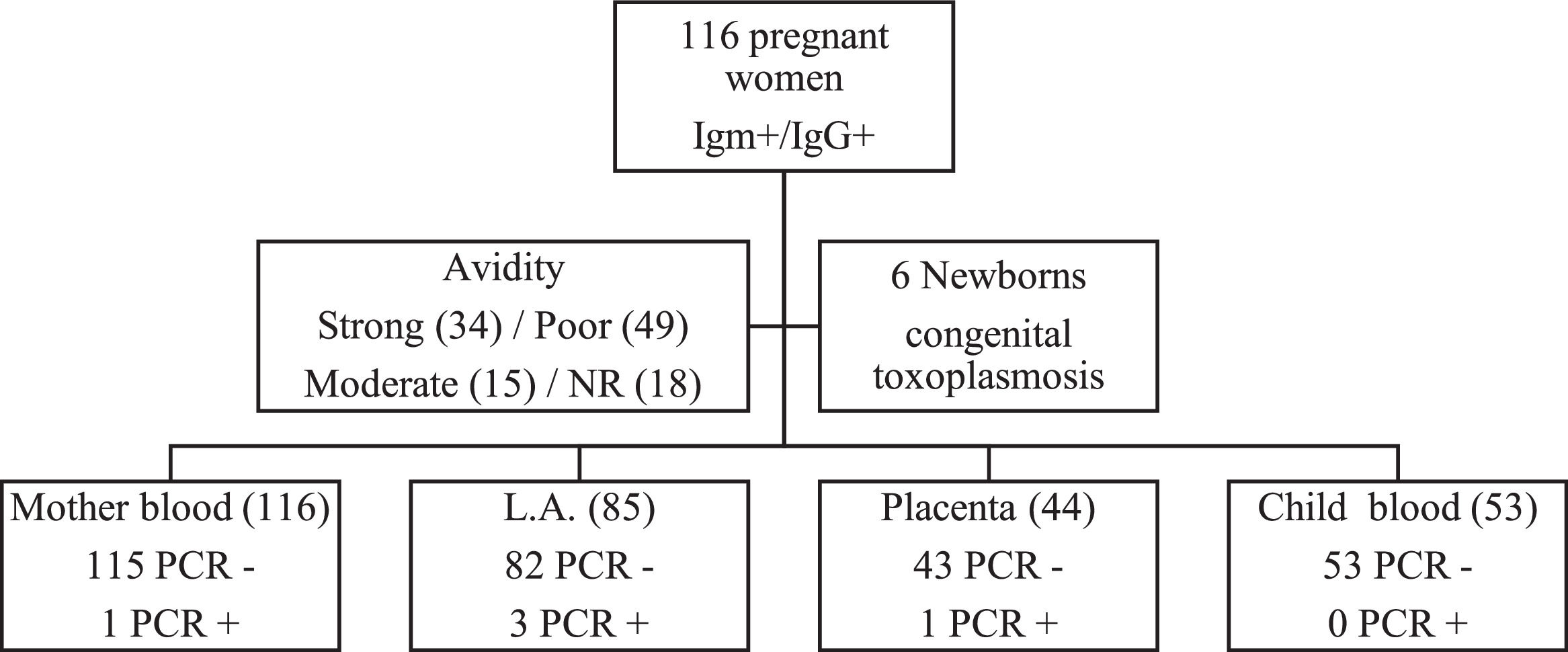
Real-Time PCR (qPCR) was performed in 298 biological samples: 116 (38.9%) of peripheral blood of pregnant women, 85 (28.5%) of amniotic fluid, 44 (14.8%) of placenta and 53 (17.8%) of peripheral blood of newborns (Fig. 1). The four samples were obtained in 33 (28.5%) cases; at least three samples in 20 (17.2%), at least two samples in 45 (38.8%) and only one sample in 18 (15.5%).
One of 116 (0.9%) pregnant women presented positive qPCR in the peripheral blood, three of 85 (3.5%) in the amniotic fluid, one of 44 (2.3%) in the placenta and no newborn (0/53) had positive qPCR in the peripheral blood. Each of these positive results occurred in different cases.
The IgG avidity test was not performed in 18 (15.5%) cases, because they presented IgG above the upper limit recommended in the manufacturer's leaflet (VIDAS®, Biomérieux, France). Thirty-four (29.3%) pregnant women presented high IgG avidity, 49 (42.3%) low and 15 (12.9%) moderate. All pregnant women with high IgG avidity were with gestational age greater than 16 weeks. The three pregnant women with positive qPCR in the amniotic fluid and the one with positive qPCR in the placenta presented low IgG avidity. However, the only pregnant woman with positive qPCR in the peripheral blood presented high avidity.
Only data for the 63 babies born in our Reference Maternity were collected. The others 53 births occurred in other maternity hospitals. The mean gestational age at birth was 38.6 weeks (minimum 30 and maximum 42), mean birth weight of 3.2 kg (minimum 1.2 and maximum 4.1) and mean head circumference of 34 cm (minimum 29 and maximum 47). The mean APGAR in the first minute was eight (minimum 5 and maximum 10) and in the fifth minute nine (minimum 7 and maximum 10).
Six (5.2%) newborns were diagnosed with congenital toxoplasmosis. Three (4.8%) newborns presented alterations suggestive of congenital infection to trans fontanelle ultrasonography and 2 (3.2%) at the ocular fundus examination. Regarding the serology result for toxoplasmosis, 3 (4.8%) presented positive anti-T. gondii IgM and 62 (98.4%) positive IgG.
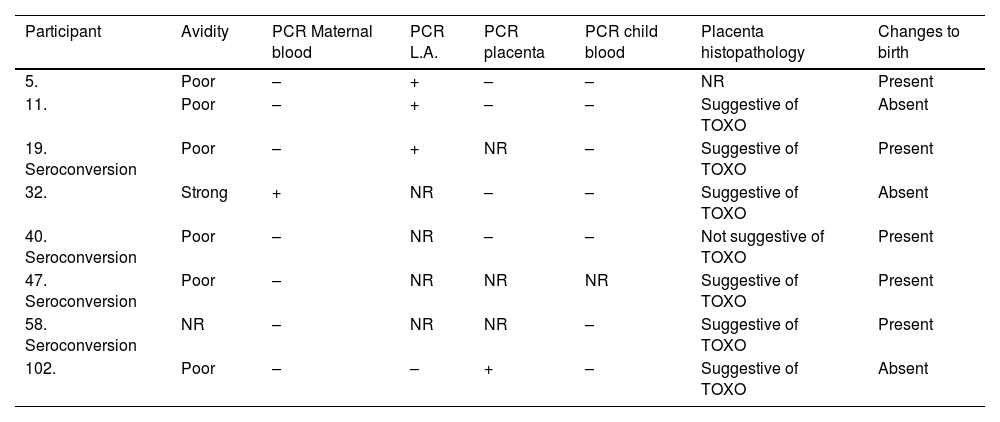
Newborns with positive RT-PCR in amniotic fluid had the diagnosis of congenital toxoplasmosis confirmed, while newborns of pregnant women with positive RT-PCR in peripheral maternal blood and placenta did not have this diagnostic confirmation (Table 1).
Description of cases of congenital toxoplasmosis and those with positive PCR sample.
NR, Not Performed; TOXO, Toxoplasmosis.
Histopathological analysis was performed in 49 placentas. Of these, 24 (49.0%) presented alterations suggestive of toxoplasmosis infection, 23 (46.9%) presented nonspecific alterations and only 2 (4.1%) were normal. It is worth mentioning that in no placenta T. gondii sample was observed. The main alterations observed were hematogenous placentitis in activity and cicatricial. The only placenta with positive RT-PCR also presented histopathological changes suggestive of toxoplasmosis.
Discussion and conclusionToxoplasmosis in Brazil is associated with important social problems such as poverty and low schooling.3 Failures in the care of pregnant women with toxoplasmosis have already been observed in previous studies12,13 and congenital toxoplasmosis persists as a serious health problem in the country.5
With the advent of molecular tests, new diagnostic possibilities for congenital toxoplasmosis emerged, with greater agility and precision.8 The high accuracy of PCR in the amniotic fluid led to its inclusion in the routine of care for pregnant women with toxoplasmosis in the reference centers and today the positive result in the amniotic fluid is one of the defining criteria of congenital disease.14 In the present study newborns with positive amniotic fluid results were diagnosed with congenital toxoplasmosis, while all with negative PCR had the diagnosis excluded.
PCR in peripheral blood has already been evaluated as an alternative for the diagnosis of toxoplasmosis. T. gondii parasitemia can be evaluated by PCR in serum, plasma, whole blood and buffy coat samples,8 the top buffy coat superior than the other samples.15 However, the sensitivity of the test in total blood samples was low (25%) in patients immunocompromised with toxoplasmosis encephalitis.16 In a study conducted in our laboratory with immunocompetent patients with acute acquired toxoplasmosis, no buffy coat sample was positive by PCR, even in more severe cases of the disease or in those where blood collection occurred very close to the onset of symptoms.17 With this, there is still doubt whether there is a significant difference in the analysis of buffy coat and total blood samples. PCR in maternal blood so far has not been shown to be promising due to its low positivity and absence of diagnostic association with infection.18 A similar result was observed among the 116 samples of maternal peripheral blood analyzed by us, being only 1 (0.9%) positive, although all pregnant women had a diagnosis of toxoplasmosis confirmed by serology.
Serological screening of infection during pregnancy allows its diagnosis, since most cases are totally asymptomatic,6 as well as symptoms were observed in only 14 (12.1%) women. Serological tests associated with IgG avidity help in the diagnosis but have limitations such as false-positive or IgG immaturity, which hinder its definition.7 The association between positive PCR in amniotic fluid and low IgG avidity, as well as strong avidity with positive PCR in maternal peripheral blood, has been described previously.18 This observation reinforces the association between low IgG avidity and positive PCR in amniotic fluid and suggests limitations of the use of the technique with maternal peripheral blood.
T. gondii presents tropism in the placenta, as well as in the central and the ocular nervous system.19 Despite this tropism, the placenta exerts an important barrier and protection function for the fetus against infectious agents.20 Therefore, the placenta is a biological material with good potential for the diagnosis of congenital toxoplasmosis. Placental tissue infection may result in placentitis,20 this alteration being the most found in histopathological analysis of all placentas that suggested diagnosis by toxoplasmosis.20 However, we found positive PCR in only one sample. To date, studies are still controversial regarding the role of the placenta in the molecular diagnosis of toxoplasmosis.8,20-22
PCR was negative in all peripheral blood samples of newborns, including those with confirmed diagnosis of congenital toxoplasmosis, this results in high positivity (48%) observed in another Brazilian study with the same methodology for molecular diagnosis.23 Different genotypes of T. gondii, more virulent strains and variations of parasitemia, may explain this difference in results.2,5
Loss of samples, mainly due to deliveries in other maternity hospitals, implying discontinuity in the follow-up of these cases and the limitation of the time of follow-up of newborns, are limitations of the study.
Deficits in the diagnostic screening of toxoplasmosis during prenatal care and difficulties in postnatal diagnosis justify the search for new diagnostic possibilities. Better understanding the role of molecular tests to assist the diagnosis of congenital toxoplasmosis using several biological specimens contributes to the future perspective of its diagnosis. However, in this study both maternal and blood samples of newborns and placenta did not prove to be promising in the diagnosis of congenital toxoplasmosis. Thus, further studies are needed to evaluate the real role of molecular diagnosis in other biological materials rather than the amniotic fluid.
Authors’ contributionsBBFV contributed to the clinical diagnosis of patients and collected samples, implemented the research, analyzed the results, and wrote the manuscript. JPPJ and CNPR clinically diagnosed patients and collected samples. DNR processed the experimental data. LHFG processed the experimental data, analyzed the results, and wrote the manuscript. ESN and LCG designed the study and were responsible for the overall direction of the project. All authors discussed the results and contributed to the final manuscript.
FundingThis research was funded by the “Research Incentives Program I (PIP I)” and approved by the Research Department of the Fernandes Figueira Institute (IFF/FIOCRUZ).
Ethics approval and consent to participateThe study was approved by the local Institutional Review Board (CAAE: 148327.13.7.0000.5269/Case Review Number: 2.482.189). Written informed consent terms was obtained from all participants in this study and from the consent of the LAR or parents or guardians of the minors. All experimental protocols in this manuscript were carried out in accordance with the ethical principles that govern research with human subjects, in accordance with the guidelines of the Declaration of Helsinki.
Availability of data and materialsThe datasets generated and/or analyzed during the current study are not publicly available due to the confidentiality and ethical aspects related to patient data but are available from the corresponding author on reasonable request.
The authors with to thank the patients and their families for participating in the study. All the experiments were developed in the technological platform “RPT04-13 ‒ PCR in Real Time ‒ IFF, belonging to the FIOCRUZ Network of Technical Platforms (PDTIS/FIOCRUZ).
Bianca Balzano De La Fuente Villar: 0000-0002-7589-354X