Febrile illnesses in developing countries are often misdiagnosed as malaria or typhoid fever. Although arboviral infections have similar clinical symptoms, they are usually not screened because of limited resources and the fact that there are several viruses in this group. Chikungunya virus (CHIKV) has been isolated in parts of Nigeria, but there is no documented evidence of the infection in Kogi State. This study determined seroprevalence of active and past CHIKV infection among febrile patients who tested negative for malaria and typhoid fever. Sera from 243 febrile patients were screened for CHIKV IgG and IgM using an immunochromatographic test kit. Clinical and socio-demographic variables were collected using a structured questionnaire. Recent CHIKV infection was observed in 5.8% of the study participants while 25.1% had IgG antibodies demonstrating previous infection. Significant associations were observed between seropositivity and age of participants (p<0.001), sex (p=0.044), marital status (p=0.002), and occupation (p<0.001). Clinical symptoms such as fever, joint pain, and headache were significantly associated with seropositivity. This study identified recent CHIKV infection in Anyigba. Therefore, there is need for routine screening of febrile patients and molecular characterization to determine the nature of circulating strains.
Chikungunya is an important re-emerging mosquito-borne disease caused by Chikungunya virus (CHIKV), an alphavirus belonging to virus family Togaviridae.1 It is vectored by Aedes species and transmitted through mosquito-to-man or man-to-mosquito interaction in urban centers. In Africa, sylvatic transmission is between these mosquitoes and non-human primates. Virus circulation is sustained in inter-epidemic periods.2 Chikungunya is an acute illness characterized by high fever, joint pain, muscle pain, and skin rash.3 It may progress to a chronic condition with debilitating arthritis, if primary infection does not resolve.4 Mothers infected during pregnancy can transmit CHIKV to their newborns5 resulting in neonatal encephalopathy, thrombocytopenia, hypotension, pericarditis, and neurologic manifestations.6 CHIKV has been identified in almost 40 countries around the world. In 2016, a total of 146,914 confirmed cases from the Americas were reported to the Pan-American Health Organization. Although the burden in many African countries is unknown, that same year 1700 suspected cases were reported from Kenya.7 Reports of CHIKV was first made in Nigeria in 1964,8 and it continues to cause significant health problems with reports of overseas importation from Nigeria.9 It is now emerging in parts of the world where it had never been reported.10 It was recently reported in Europe and USA, raising concerns of associated risk following increased international travel to endemic areas of the world.11 Other factors that facilitate spread includes trade in products with vector larvae, climate change which supports vector survival, and lack of herd immunity.12 In developing countries such as Nigeria, malaria or typhoid is the tentative diagnosis for most febrile illnesses. Clinicians seldom consider arboviral infections, despite the fact that they produce clinically similar symptoms.13 Consequently, there is underreporting, increased morbidity and mortality from arboviral infections.14 There is currently no information on CHIKV infection in Kogi State. Nigeria is the most populous country in Africa, and Kogi State is the gateway to Northern and Southern parts of the country with usually heavy vehicular traffic as people transit the nooks and crannies of the nation, including international visitors. This study determined seroprevalence of antibodies against previous and current CHIV infection to provide preliminary data and inform policy decisions for environmental vector control and health promotion. It also provides socio-demographic and clinical tools to guide differential diagnosis of these arbovirosis.
MethodologyStudy designIn this cross-sectional study, febrile participants were enrolled by random sampling from five hospitals in Anyigba - Dekina Local Government of Kogi State, Nigeria. Population of Anyigba is about 18,907 and it is located in the tropical hinterland climate with geographical coordinate 70121E, 70,361N.15 Patients were enrolled from Kogi State University Teaching Hospital (36), Maria Goretti (75), Christ the Good Shepherd Hospital (40), Grimard Catholic Hospital (3) and Peace Hospitals (39). A total of 243 patients with undifferentiated febrile illness were enrolled from February to July 2018. Participants aged ≥ 18 years provided written informed consent. For consenting attendees aged 5–17 years, parents or legal guardians provided written informed consent. Trained medical personnel in each hospital explained the objectives of the study before consenting patients with symptoms of CHIKV infection such as fever, joint pain with or without abdominal pain, vomiting, headache, skin rash, and nausea were recruited. Exclusion criteria included patients with fever or pain related to diabetes mellitus, rheumatic diseases and HIV/Aids. Patients under five years of age were excluded. Ethical approval for the study was obtained from Institutional Review Board (IRB) of Kogi State University Teaching Hospital in accordance with Code of Ethics of World Medical Association Declaration of Helsinki. Sample size (N) was determined using formula N=Zα2pq/d2 3, where Zα = standard normal deviate set at 1.96, corresponding to 95% confidence interval (95% CI); p=proportion in the target population estimated to have a variable characteristic=41.8% (0.418) from similar study in Nigeria16; q=1 - p=58.2% (0.582); and d=degree of precision set at 0.05 (95% CI). For logistic correlation, 243 serum samples were randomly selected for this study. A structured questionnaire was used to obtain socio-demographic data and clinical variables of patients.
Sample collection and processingFive milliliters of venous blood was collected into sterile sample bottle. Serum were separated by centrifugation at 4000rpm for five minutes with GreatMed® centrifuge and stored at −20°C until tested.
IgG and IgM assay against chikungunya virusCHIV IgG/IgM detection kit was purchased from Biopanda Diagnostic and Reagents Limited® (United Kingdom). This assay detects anti-CHIV IgG and IgM antibodies which bind to conjugated recombinant CHIV antigen on nitrocellulose membrane. Chromatographic migration on membrane occurs by capillary action to react with mouse anti-human IgG and/or mouse anti-human IgM on membrane to produce coloured line which is indicative of positive result. The third coloured line serves as positive control which validates the assay.
Data analysisData from this study was analyzed with Statistical Packages for Social Sciences (SPSS) version 16.0 for windows (SPSS Inc., Chicago, IL, USA). Descriptive data are presented in tabular forms. Chi-square test and logistic regression was used to test for association between test variables and prevalence rates. Odd ratios (OR) was computed at 95% confidence intervals (CI) to determine strength of association between seropositivity and variables. Level of statistical significance was set at p≤0.05.
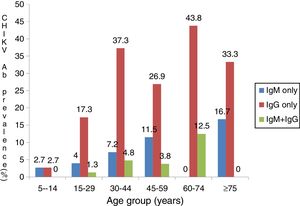
ResultsThere were 110 (45.3%) males and 133 (54.7%) females between five and 80 years of age. Participants were mostly in the age-range of 30–44 years (34.2%) while 2.5% were in age group 75–80 years (Table 1). Out of 243 febrile participants screened, 14 (5.8%) had anti-CHIKV IgM, 61 (25.1%) had anti-IgG and 8 (3.3%) had both IgG and IgM. Overall prevalence of 34.2% (83/243) was observed (Table 1). IgM and IgG seropositivity was significantly associated with male sex (p=0.044, X2=4.075). Males had almost two-fold greater chance of contracting CHIKV compared to females (OR=1.7, CI=1.014–2.955). CHIKV seropositivity increased with age,a s shown in Fig. 1 (p<0.001). Active CHIKV infection was found in all age groups, but more common in people older than 75 years. Active infection was lowest in age group 5–14 years. Dual seropositivity was more observed in married people compared to divorced, widowed and single individuals (p=0.002; Table 2). With exception of those with primary level of education, there was increasing trend in seropositivity as educational level increased. Farmers had higher prevalence of current infection (48.1%), followed by people who engaged in business activities. Significant association was observed between participant’s occupation and active infection (p<0.001). Insecticide users had higher seropositivity (41.6%) compared to non-users (29.2%). Fever (10.8%), abdominal pain (10.8%), and diarrhea (9.5%) were associated with high IgM levels and dual (IgG and IgM) seropositivity. However, this was not statistically significant (Table 3). Significant association was observed between joint pain and IgG seropositivity (p=0.042). Participants who were confirmed with malaria were twice more likely to have been previously exposed to Chikungunya (p=0.033, OR=2.0, CI: 1.051–3.997).
Anti-CHIKV antibodies and its distribution in relation to sex, age and location of study participants.
| Variable | No. of Participant | Proportion %) | No.%) of specific anti-CHIKV-Abs | Total anti-CHIKV-Abs | |||||
|---|---|---|---|---|---|---|---|---|---|
| IgM only | IgG only | IgM+IgG | |||||||
| Gender | |||||||||
| Male | 110 | 45.3 | 8 (7.3) | 32 (29.1) | 5 (4.5) | 45 (40.9) | |||
| Female | 133 | 54.7 | 6 (4.5) | 29 (21.8) | 3 (2.3) | 38 (28.6) | |||
| Total | 243 | 100 | 14 (5.8) | 61 (25.1) | 8 (3.3) | 83 (34.2) | |||
| X2 | 4.075 | ||||||||
| P-value | 0.044 | ||||||||
| Age | |||||||||
| 5–14 | 37 | 15.2 | 1 (2.7) | 1 (2.7) | 0 (0.0) | 2 (5.4) | |||
| 15-29 | 75 | 30.9 | 3 (4.0) | 13 (17.3) | 1 (1.3) | 17 (22.7) | |||
| 30–44 | 83 | 34.2 | 6 (7.2) | 31 (37.3) | 4 (4.8) | 41 (49.4) | |||
| 45-59 | 26 | 10.7 | 3 (11.5) | 7 (26.9) | 1 (3.8) | 11 (42.3) | |||
| 60–74 | 16 | 6.6 | 0 (0.0) | 7 (43.8) | 2 (12.5) | 9 (56.3) | |||
| 75–80 | 06 | 2.5 | 1 (16.7) | 2 (33.3) | 0 (0.0) | 3 (50.0) | |||
| Total | 243 | 100 | 14 (5.8) | 61 (25.1) | 8 (3.3) | 83 (34.2) | |||
| X2 | 31.485 | ||||||||
| P-value | 0.000 | ||||||||
| Location | |||||||||
| KSUTH | 36 | 14.8 | 0 (0.0) | 13 (36.1) | 0 (0.0) | 13 (36.1) | |||
| MGH | 75 | 30.9 | 3 (4.0) | 19 (25.3) | 2 (2.7) | 24 (32.0) | |||
| PH | 39 | 16.0 | 2 (5.1) | 5 (12.8) | 4 (10.3) | 11 (28.2) | |||
| GCH | 3 | 21.8 | 6 (11.3) | 11 (20.8) | 0 (0.0) | 17 (32.1) | |||
| CGCH | 40 | 16.5 | 3 (7.5) | 13 (32.5) | 2 (5.0) | 18 (45.0) | |||
| Total | 243 | 100 | 14 (5.8) | 61 (25.1) | 8 (3.3) | 83 (34.2) | |||
| X2 | 3.02 | ||||||||
| P-value | 0.55 | ||||||||
Key: KSUTH=Kogi State University Hospital; MGH=Maria Gorethi Hospital; PH=Peace Hospital; GCH=Grimard Catholic Hospital, CGCH=Christ the Good shepherd Hospital; Abs=Antibodies; X2 = Chi square value.
Socio-demographic characteristics of patients with active/recent and previous CHIKV infection.
| Variable | No. Tested | Total No. (%) of anti-CHIKV-Abs | P-value | No. (%) of IgM/ IgM+IgG positive | P-value | No. (%) of IgG positive | P-value |
|---|---|---|---|---|---|---|---|
| Educational level | |||||||
| None | 40 | 13(32.5) | 0.43 | 4 (10.0) | 0.99 | 9 (0.23) | 0.30 |
| Primary | 32 | 7 (21.9) | 3 (9.4) | 4 (0.13) | |||
| Secondary | 82 | 30 (36.6) | 7 (8.5) | 23 (0.28) | |||
| Tertiary | 89 | 33 (37.1) | 8 (9.0) | 25 (0.28) | |||
| Marital status | |||||||
| Married | 112 | 51 (45.5) | 13 (11.6) | 38 (0.34) | |||
| Divorced | 16 | 7 (43.8) | 0.002 | 2 (12.5) | 0.49 | 5 (0.31) | 0.01 |
| Widowed | 22 | 6 (27.3) | 1 (4.5) | 5 (0.23) | |||
| Single | 93 | 19 (20.4) | 6 (6.5) | 13 (0.14) | |||
| Occupation | |||||||
| Farming | 52 | 25 (48.1) | 8 (15.4) | 17(0.33) | |||
| Civil servants | 39 | 15 (38.5) | 0.000 | 2 (5.1) | 0.25 | 13 (0.33) | 0.003 |
| Business | 59 | 26 (44.1) | 6 (10.2) | 20 (0.34) | |||
| Students | 93 | 17 (18.3) | 6 (6.5) | 11 (0.12) | |||
| Insecticide use | |||||||
| Yes | 113 | 47 (41.6) | 13 (11.5) | 0.26 | 34 (0.30) | 0.10 | |
| No | 130 | 38 (29.2) | 0.023 | 9 (6.9) | 27 (0.21) |
Clinical manifestations in patients with anti-CHIKV IgM antibody.
| Symptoms | No. Tested | No. (%) positive | Odd ratio (OR) | 95% CI | P-value |
|---|---|---|---|---|---|
| Fever | |||||
| Yes | 194 | 15 (7.7) | |||
| No | 49 | 7 (14.3) | 0.5 | 0.193–1.311 | 0.153 |
| Rash | |||||
| Yes | 15 | 0 (0.0) | |||
| No | 228 | 22 (9.6) | – | – | 0.207 |
| Nausea | |||||
| Yes | 67 | 3 (4.5) | |||
| No | 176 | 19 (10.8) | 0.4 | 0.111–1.354 | 0.125 |
| Headache | |||||
| Yes | 169 | 14 (8.3) | |||
| No | 74 | 8 (10.8) | 0.7 | 0.298–1.861 | 0.528 |
| Joint pain | |||||
| Yes | 124 | 10 (8.1) | |||
| No | 119 | 12 (10.1) | 0.8 | 0.325–1.885 | 0.583 |
| Abdominal pain | |||||
| Yes | 97 | 10 (10.3) | |||
| No | 146 | 12 (8.2) | 1.2 | 0.532–3.099 | 0.578 |
| Diarrhea | |||||
| Yes | 42 | 4 (9.5) | |||
| No | 201 | 18 (9.0) | 1.1 | 0.343–3.341 | 0.907 |
| Fever +Headache | |||||
| Yes | 141 | 12 (8.5) | |||
| No | 102 | 10 (9.5) | 0.9 | 0.355–2.065 | 0.729 |
| Fever +Joint Pain | |||||
| Yes | 106 | 7 (6.6) | |||
| No | 137 | 15 (10.9) | 0.6 | 0.226–1.466 | 0.242 |
| Fever +Abdominal Pain | |||||
| Yes | 83 | 9 (10.8) | |||
| No | 160 | 13 (8.1) | 1.4 | 0.562–3.364 | 0.484 |
High IgG seropositivity obtained in this study indicates Chikungunya is endemic in Kogi State. Together with few reports from other parts of Nigeria, findings from our study underscore the fact that mosquitoes spread easily across parts of Nigeria. The derived Savannah where Kogi State is located serves as a buffer zone. It is possible that this area sustains CHIKV for onward transmission to the arid northern part, as visitors on transit are bitten by the vectors. Dual IgG and IgM positivity observed among participants in this study indicates ongoing infection despite previous exposure. Both immunological markers translate past and ongoing or acute infections,17,18 suggesting CHIKV is maintained at an endemic threshold within the area. Age was found to be an important factor for active CHIKV infection, as shown in Fig. 1. Higher rates observed in age group 60–74 years is in agreement with previous reports among older patients in Chandrapur and Singapore.19,20 In those reports, direct relationship was found between increasing age and susceptibility to CHIKV. This is a result of anatomical and functional changes, degree of exposure, and impaired immune functions especially among the geriatric population.19 Furthermore, elderly people maintain sedentary lifestyles as they sit for long periods in unscreened places, thereby being exposed to Aedes species. Males were more exposed to CHIKV in the present study, despite having more febrile females visiting the health facilities (Table 1). This sex bias has been previously reported20–22 and may be attributed to cultural habits and behaviours which predispose males to more bites by Aedes species. Men are more adventurous; they hunt and fend for the family, irrespective of the place and time. They enter forested areas, swamps, and may not protect themselves from mosquito bites. Higher seroprevalence observed among patients with tertiary education may be due to increased outdoor physical activity or sedentary lifestyle in places infested by Aedes species without protection against vectors. Active infection was observed to occur more in married participants (Table 2). This has been corroborated by studies in two south-western states in Nigeria.16 Couples are usually in close proximity compared to widows or widowers, if one partner acquires infection the other partner can be horizontally infected following blood meal by vector. Indeed, transmission can affect their children which by implication is the single or unmarried person living in the household. Incidentally, infected mosquito can easily spread CHIKV to family members. Working on farms and agricultural fields was associated with higher CHIKV seropositivity (Table 2). This was also observed in Chandrapur, India23 and may be the result of frequent exposures while on their farmlands. As water collects in poodles or from watering crops, there is increased mosquito activity which predisposes farmers to bites by Aedes species.
Fever, abdominal pain and dysentery are predominant acute symptoms associated with CHIKV in this study. Similarly, same symptoms were previously observed among people in India.24 Joint pain was significantly associated with past CHIKV infection. This agrees with reports in Tanzania and Ethiopia where joint pain was a common finding among people with CHIKV.25,26 Some participants were co-infected with malaria and CHIKV in this study. Both are mosquito-blood-borne infections and not mutually exclusive. This is why it is very difficult to distinguish clinically between malaria and arboviral infections, leading to misdiagnoses. Immunosuppressive nature of malaria27 may lead to increased risk of acquiring active CHIKV infection. A limitation in our present study is that only symptomatic patients were considered, whereas Chikungunya infection may be asymptomatic. Thus, it is possible to detect both antibodies in apparently healthy population.
In conclusion, this study shows CHIKV is actively circulating in Anyigba. It highlights the need to rule out differential causes of arboviral infections. This is the first CHIKV serosurveillance in Kogi State. If control measures are not instituted, it portends major health threat because it is located in the middle belt which serves as transit point for travellers from north and south. It reinforces inclusion of CHIKV surveillance and routine screening for other arboviruses in febrile patients. CHIKV infection requires prompt attention because there is currently no vaccine. There is need for vector control strategies, intensive awareness creation through media, evacuation of gutters and clearing of bushes.
Conflicts of InterestThe authors declare no conflicts of interest.
FundingNo specific funding was received for this project.
We appreciate the support and cooperation of management of Kogi State University Teaching Hospital, Maria Goretti, Peace, Grimard and Christ the Good Shepherd hospitals. We thank the participants for their willingness to be enrolled in the study.