To describe the prevalence and factors associated with serologic response to Listeria monocytogenes in HIV infected and uninfected pregnant women in Brazil.
MethodsCross-sectional study, pregnant women after 14 weeks of gestational age were enrolled. Positive serologic test for L. monocytogenes was defined as titers >1:80 (agglutination test). Comparisons were performed using logistic regression.
ResultsA total of 213 women were enrolled, 73 (34%) were HIV infected. 55 women were seroreactive for L. monocytogenes, 27 (37%) HIV-infected and 28 (20%) HIV-uninfected (p < 0.01). Considering the diet record, white cheese consumption was associated with seroreactivity (p < 0.01). In the group of pregnant women living with HIV, the variables associated with L. monocytogenes positive serology were: lower CD4+ cells count at study entry OR=4.8 (95%CI=1.1–19.8) and having neonates admitted to the intensive care unit OR=5.9 (95%CI=1.01–34.9).
ConclusionPositive serology for Listeria monocytogenes was associated with HIV infection. Brazilian women should avoid white cheese during pregnancy.
Listeria monocytogenes is a foodborne pathogen. Outbreaks of human listeriosis are associated to a wide variety of food consumption: dairy products (mainly white or soft cheeses), meat and ready-to-eat products (processed foods, salads, raw vegetables, sausages).1–5
Listeriosis usually occurs as a result of T-cell-mediated immunodeficiency failing to control the proliferation and invasion of L. monocytogenes in the GIT (gastrointestinal tract).6 In this context, individuals with T-cell-mediated immunodeficiency, as people living with HIV, are a risk group for listeriosis.
Pregnant women are more vulnerable to L. monocytogenes infection and are up to 20 times more likely to develop listeriosis, probably due to hormonal and immunological changes that occur during this period, especially in the third trimester.7 Although all these immunosuppressive conditions present a potentiated risk for developing listeriosis, the common interaction between pregnancy and HIV infection was not studied.8,9
Socioeconomic and demographic factors such as age, income, education, and ethnicity are associated with listeriosis during pregnancy. Ethnic origin other than Caucasian and low income are variables described as risk factors for listeriosis during pregnancy in developed countries. Cultural differences related to eating habits, health care, and hygiene may also impact the incidence of listeriosis.6,10–12
The prevalence and risk factors for listeriosis were less studied in developing countries.
The aim of this study was to assess the prevalence and possible factors associated with the serologic response to L. monocytogenes, considering HIV specific risk factors and pregnancy outcomes.
Materials and methodsRecruitmentThis was a cross-sectional study, from 2014 to 1016. Pregnant women, in the second or third trimester of pregnancy who were approached during their antenatal visits and accepted to participate in this study were included in the study. Pregnant women living with HIV (PWLH), HIV, defined based on the Brazilian Health Ministry algorithm: two different samples from different tests (serology, rapid test, or molecular based test) positive for HIV, were followed up at the Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG), a reference center for the care of PWLH, affiliated to the Universidade Federal do Rio de Janeiro. HIV-uninfected pregnant were enrolled in primary care clinics in Petrópolis (a city near Rio de Janeiro). Women who accepted to participate were interviewed and blood samples were collected. Those women who did not understand and sign the informed consent, and those using antibiotics at enrollment were excluded.
Demographic information, socioeconomical, clinical (use of antibiotics and antiretrovirals), and laboratory data (CD4+ cells count, in cells/mm3 and HIV-1 viral load, in copies/mL at 34 weeks of gestational age) were collected through a structured questionnaire. The participants also filled out a dietary record about the three days prior to the interview. The group of PWLH were followed up during the puerperal period: after delivery, the HIV pregnant women returned to IPPMG antenatal clinic, to be discharged and to register their neonate in the HIV exposed well baby clinic. In this visit, we collected data from labor and delivery, as well as the neonatal period. The pregnancy outcomes, and neonatal characteristics were also evaluated. Neonate gestational age was calculated based on Capurro score.13
Serology tests for anti-L. monocytogenesAll samples were processed at the Laboratório de Zoonoses Bacterianas (LABZOO) from Instituto Oswaldo Cruz (IOC), Fundação Oswaldo Cruz (Fiocruz), within 24h after collection.
The blood samples were collected in sterile tubes with coat activator and kept under refrigeration (4–8 °C) for complete clot retraction, followed by separation of the serum by centrifugation. The serum was transferred to sterile tubes and stored under -20 °C until carrying out the assays, performed periodically in batches.
A screening of 213 blood serum samples were performed by slide agglutination test, making a dense suspension (0.5% phenolic saline) of L. monocytogenes OH antigens of serotypes 1/2a and 4b, according to Gray and Killinger.14
All seroreactive samples in the previous test were analyzed with slow agglutination (Widal test) using the same OH antigens, diluted to 3.0 Mc Farland scale. The positive titers were the highest dilution of serum with visible and total agglutination, classified as: reactive (titers > 1:80, according to Seeliger and Hohne).15
Statistical analysisAll data were stored on Excel 16.0 spreadsheets and exported to STATA 13.1 (Texas, EUA).
The distribution of the continuous variables was described according to the measures of central tendency (median) and dispersion (interquartile range-IQR), while the categorical variables were summarized by frequency measures. CD4 cells count and HIV viral load were reported as continuous and categorical variables, categorized in < or ≥ 350 cells/mm3 and < or ≥ 40 copies/mL, respectively. The main dependent variable in these analyses was the serologic response to L. monocytogenes, defined as titers > 1:80 on slow agglutination test. Univariate analysis was performed using Fisher's exact test (categorical variables) and Mann-Whitney test (continuous variables). Independent variables with p-value <0.2 were included in a multivariate analysis model, using backwards stepwise logistic regression. Models with and without interactions were tested using the -2 log-likelihood test. The fitness of the model was evaluated using the Hosmer Lemeshaw test. Alternative hypothesis was accepted if p-value is ≤ 0.05 (this analysis were conducted for the whole group, and within the PWLH group).
Ethical considerationsThe study was approved by the Research Ethics Committee of the Instituto de Puericultura e Pediatria Martagão Gesteira (IPPMG) of UFRJ under CAAE no. 608.313. All patients signed the informed consent, in order to participate on the study.
ResultsFrom 2014 to 2016, a total of 213 pregnant women were included in the study: 73 HIV-infected (all on antiretrovirals at study entry) and 140 HIV-uninfected. Overall median age was 27 years old (interquartile range-IQR: 22–33), and median gestational age at the study entry of 26 weeks (IQR=19–32).
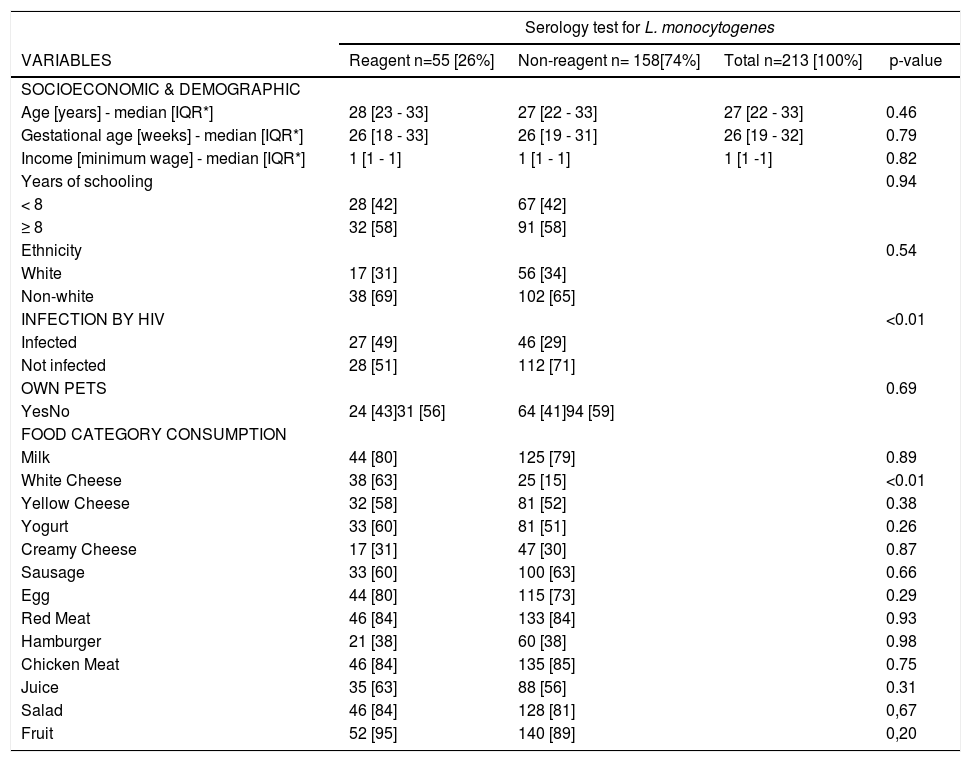
A total of 90 (42.3%) women were seroreactive for L. monocytogenes on slide agglutination test. Combining the results of 1/2 and 4b antigens on agglutination test, 55 women turned out positive: 27 (37%) HIV-infected women and 28 (20%) HIV-uninfected (p-value <0.01) (Table 1).
⁎⁎ Socioeconomic, clinical and dietary record variables associated with anti-L. monocytogenes positivity in serum of pregnant women.
* IQR [interquartile range].
⁎⁎No missing data in this Table.
When analyzing the results from the slide agglutination test according to the antigen used, 39 samples were reactive to antigen 1/2, with 17 (63%) HIV-infected patients and 22 (79%) HIV-uninfected (p-value = 0.20). Similar rates were found among those reactive for 4b antigen: 23 (85%) HIV infected and 23 (82%) HIV-uninfected (p-value = 0.76).
In the dietary questionnaire, consumption of white cheese was associated with reactive serology for L. monocytogenes (Table 1).
In multivariate analysis, the only food category independently associated with positive serology for L. monocytogenes was also white cheese [odds ratio=2.65, 95% confidence interval (95%CI)=1.38–5.05].
In other multivariate analysis, after adjusting for socioeconomic (SES) variables (income and number of years of education), the odds ratio for the association between HIV infection and seroreactivity for L. monocytogenes was 2.34 (95% CI 1.22-4.46, p < 0.01). There were no significant differences in SES variables between HIV-infected and uninfected patients.
From the 73 PWLH initially enrolled, 67 (92%) could be followed until delivery. There was one fetal death whose mother´s serology reaction for L. monocytogenes was negative. At study entry, their median CD4 cells count was 488, and the IQR was 309–673 cells/mm3.
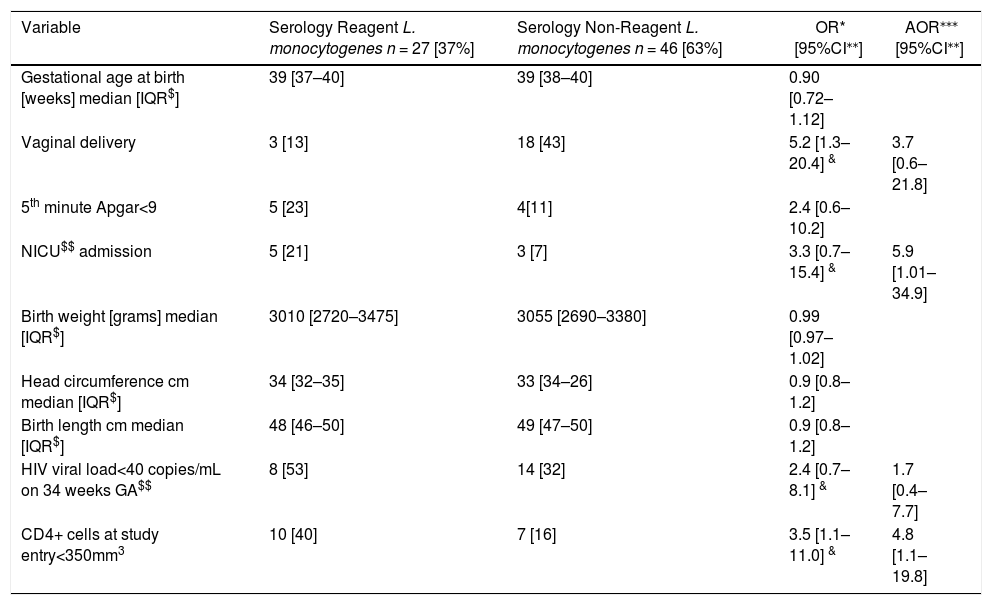
Indeed, PWLH who presented reactive serologic test for L. monocytogenes were more immunosuppressed at the study entry. After adjusting for CD4+ cells count <350/mm3 at study entry, non-vaginal delivery mode, and HIV viral load <40 copies/mL at delivery, babies born to those mothers had higher chance to be admitted to the neonatal intensive care unit at birth (Table 2).
Laboratory and neonatal characteristics of HIV-infected pregnant women.
| Variable | Serology Reagent L. monocytogenes n = 27 [37%] | Serology Non-Reagent L. monocytogenes n = 46 [63%] | OR* [95%CI⁎⁎] | AOR⁎⁎⁎ [95%CI⁎⁎] |
|---|---|---|---|---|
| Gestational age at birth [weeks] median [IQR$] | 39 [37–40] | 39 [38–40] | 0.90 [0.72–1.12] | |
| Vaginal delivery | 3 [13] | 18 [43] | 5.2 [1.3–20.4] & | 3.7 [0.6–21.8] |
| 5th minute Apgar<9 | 5 [23] | 4[11] | 2.4 [0.6–10.2] | |
| NICU$$ admission | 5 [21] | 3 [7] | 3.3 [0.7–15.4] & | 5.9 [1.01–34.9] |
| Birth weight [grams] median [IQR$] | 3010 [2720–3475] | 3055 [2690–3380] | 0.99 [0.97–1.02] | |
| Head circumference cm median [IQR$] | 34 [32–35] | 33 [34–26] | 0.9 [0.8–1.2] | |
| Birth length cm median [IQR$] | 48 [46–50] | 49 [47–50] | 0.9 [0.8–1.2] | |
| HIV viral load<40 copies/mL on 34 weeks GA$$ | 8 [53] | 14 [32] | 2.4 [0.7–8.1] & | 1.7 [0.4–7.7] |
| CD4+ cells at study entry<350mm3 | 10 [40] | 7 [16] | 3.5 [1.1–11.0] & | 4.8 [1.1–19.8] |
In this study we showed that 26% in this recruited population had a positive serological response to L. monocytogenes. PWLH had a higher chance to be seroreactive to L. monocytogenes than HIV-uninfected women, even adjusting to SES variables. The main dietary habit associated with L. monocytogenes seropositivity was consumption of white cheese.
In contrast to phenotyping or genotyping detection, serologic studies have not demonstrated GIT carriage of L. monocytogenes, but rather a previous infection/carriage able to induce immune response to this microorganism. As previously reported, PWLH are more susceptible to listeriosis and it was confirmed, considering the fact that they have higher prevalence of serologic response. In addition, lower socioeconomical status (SES) is also related to listeriosis.7,8,12 In order to minimize the possible bias in the association between HIV infection and L. monocytogenes serologic response, we adjusted for SES variables (income and study years) in the multivariate analysis, and the higher prevalence in PWLH remained significant.
Another important point is the possibility of false positive serologic reaction for L. monocytogenes. This serologic test may present cross-reaction with other Gram-positive microorganisms from GIT microbiota, including Staphylococcus spp, Streptococcus spp, Enterococci, and Bacillus spp.14,15 Indeed, L. monocytogenes presents an heterophile antigen Rantz in its surface, which is also present as a surface antigen in other Gram-positive microorganisms. Although, the Rantz antigen may cause cross-reactive serological response to L. monocytogenes, the titers observed are up to 1:40, and we considered as immune response to L. monocytogenes titers ≥1:80.16 Titers ≥1:320 are associated from acute listeriosis.17 None of our patients had L. monocytogenes titers >1:320, and all were asymptomatic.
Pregnant women who consumed white cheese were more likely to have positive serology for L. monocytogenes, although, there is no temporal association between the 3-day-recordatory diet questionnaire and the serological response. The assumption that the dietary habits would not change overtime, should be considered. Also corroborating our findings, several authors verified that consumption of dairy products were among the foods most consumed by pregnant women diagnosed with listeriosis.18,19 Considering the geographical differences of dietary habits, it is important to identify the main food groups probably associated with listeriosis in a middle-income country from Latin America.
Among PWLH, those with reactive serologic test for L. monocytogenes had lower CD4+ cells count at study entry. Even after adjusting for delivery mode, immunosuppression, and undetectable HIV viral load at 34 weeks of gestational age, neonates born to mothers with reactive serology for L. monocytogenes were more likely to be admitted to the NICU at birth than neonates born to non-reactive mothers. The reason for this finding must be better elucidated in subsequent studies, considering the reason for the NICU admission. However, this trend was maintained after adjusting for birth weight or gestational age at birth.
In conclusion, PWLH presented higher seropositivity for L. monocytogenes. These findings confirm that HIV infection should be considered a risk factor for listeriosis in a middle-income country, such as Brazil, although, since we used serologic tests, the temporality between HIV and Listeria monocytogenes infection could not be determined.
There was an association between consumption of white cheese and seropositivity for L. monocytogenes. This result is important not only individually, but also considering the public health perspective. People living with HIV, and mainly pregnant women must be counselled to avoid eating white cheese.
Among PWLH, those with lower CD4 cells count at study entry had higher odds to have a positive serology for L. monocytogenes, and their neonates higher odds to be admitted to the NICU. Studies with longer follow up, and larger sample size must be pursued, in order to better understand the reasons for this last finding.
This work was supported by CNPq – (grant numbers: 306699/2014-1 for EH and 304476/2018-8 for CBH) and CAPES – for IGRF.