Clinical improvements following highly active antiretroviral therapy (HAART) may increase high-risk behaviors resulting in sexually transmitted infections (STI). Optimism related to the success of HAART in slowing disease progression, reducing viral load, and improving health status might be important factors for increasing sexual risk behaviors such as less use of condoms.
ObjectiveTo determine the prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis, hepatitis B and C, high-risk HPV, and cervical cytological abnormalities among women living with HIV (WLHIV) who attended a Reference Center for STI/AIDS in Brazil.
MethodsA cross-sectional study was conducted among 151 WLHIV attending an STI Clinic in Vitória city, Brazil. A structured questionnaire, including demographic, behavioral, and clinical information, was used for data collection. Serological tests for HIV, syphilis, hepatitis C and B, CD4 counts, and viral load determination were performed. Cervical samples were collected for cytology and real-time PCR for HPV,Chlamydia, and Neisseria gonorrhoeae.
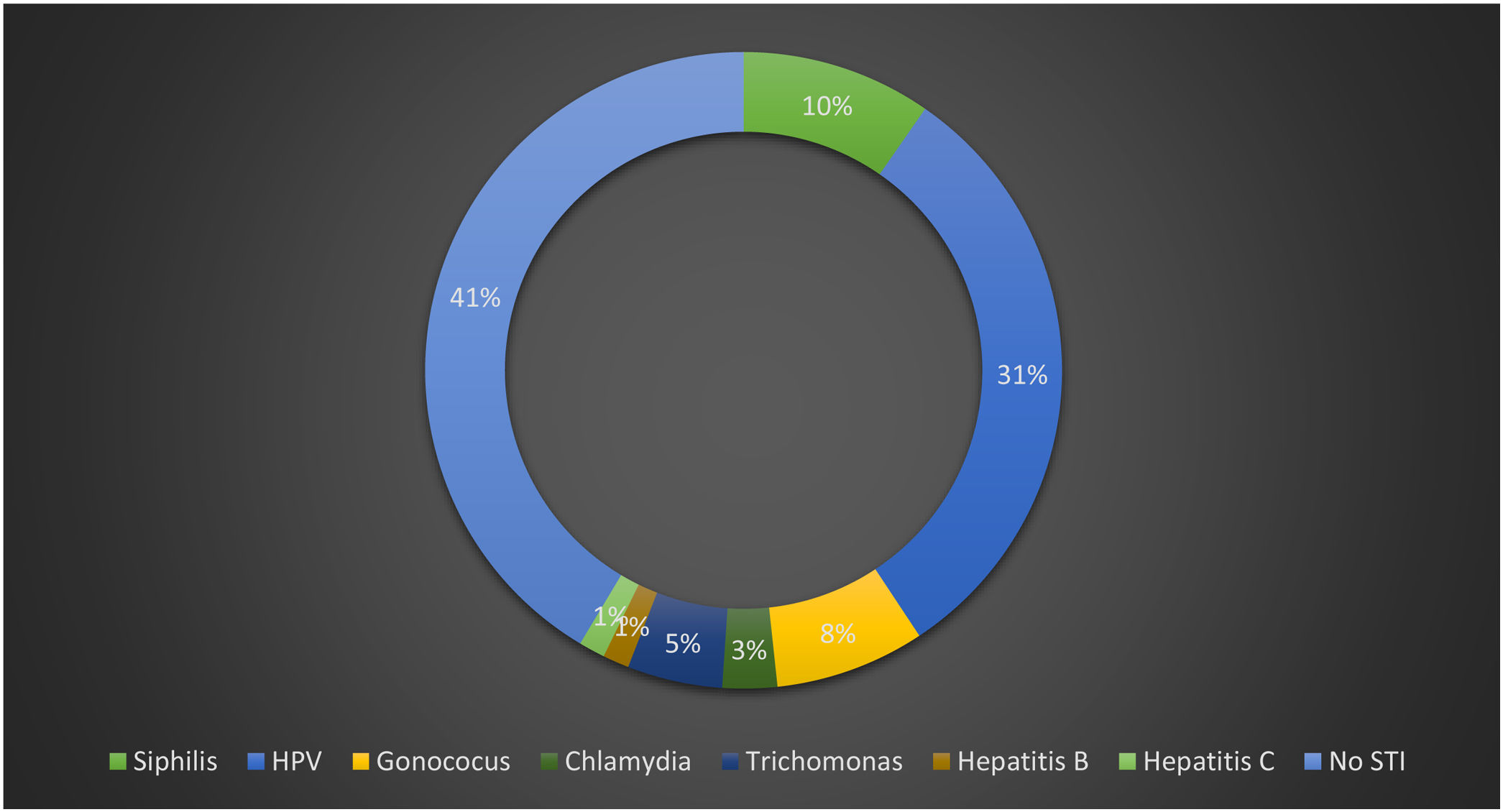
ResultsIn this study, 59% of women had at least one diagnosed STI at the time of the first clinic visit; 31% had clinical forms of anogenital HPV, 10% syphilis, 8%Neisseria gonorrhoeae, 5.0% trichomoniasis, 3% Chlamydia trachomatis, 1% hepatitis B, and 1% hepatitis C; 6.7% of the women presented with cervical cytological abnormalities. Furthermore, 46.3% of women had HR-HPV, and 17.6% had HPV 16/18. Only 5% of the women had a CD4 count <200 cells/mm3, 61.6% had undetectable HIV viral load, and 81.3% were currently on HAART.
ConclusionA high prevalence of STI and HR-HPV infections were observed among HIV-infected women in this investigation. Prevention programs need to focus on counseling WLHIV and their regular partners with focused interventions such as couples counseling and education programs.
Prevalence and incidence data are important information in the design and evaluation of programs and interventions for sexually transmitted infections (STIs) and in interpreting changes in HIV epidemiology. The World Health Organization (WHO) periodically generates estimates to assess the global burden of four of the most common curable sexually transmitted infections.1 Strategies for preventing and controlling HIV infection based on behavioral risk factors can be improved by aggregating information about biological determinants that contribute to HIV transmission. From a policy perspective, treatment of curable STIs is an essential part of primary health care and should continue to be promoted as one component of HIV control programs in communities in which the burden of STIs is substantial.2 In addition, bacterial vaginosis is associated with increased risk of multiple adverse outcomes, including transmission and acquisition of human immunodeficiency virus type 1 (HIV-1) and STIs.3 Furthermore, HIV-infected persons may have a modified clinical course of STIs owing to decreased immunity, leading to poor quality of life and increased morbidity and mortality.4
The development of highly active antiretroviral therapy (HAART) has led to a reduction in mortality and morbidity rates among people living with HIV (PLHIV).1,2 Early initiation of HAART will lead to near-normal quality of life and lifespan5 and reduce plasma viral load, which is a primary determinant of the risk of transmission.5,6 Moreover, reduction of transmission to sexual partners among those initiating HAART will depend upon viral load in plasma and genital tract.1,7 Although rare, even with the infected partner on effective HAART and undetectable viral load, HIV may still be transmission has been documented.7,8 Thus, the risk is substantially reduced, but it is not completely eliminated. Rapid development in the field of HAART has led to a reduction in morbidity and mortality among PLHIV. There is a growing concern over sexual risk behavior (multiple partners or unprotected sex) of PLHIV on HAART. Changes in sexual risk behavior of PLHIV after initiation of HAART might have important implications on the way counseling may be designed and implemented. Increased sexual risk behavior with therapy will be a matter of great concern from the perspective of preventing transmission. If not intervened, it may further fuel the epidemic.9 Optimism related to the success of HAART in slowing disease progression, reducing viral load, and improving health status (significant improvements occurred across general health perception; physical, social, and cognitive functioning; pain; mental health; energy; and fatigue) might be crucial factors determining sexual risk behaviors.10,16
Being HIV-infected is hypothesized to be a sufficiently dramatic experience to destroy any unfounded belief in the effectiveness of the strategy of protection.¹¹
The study on the prevalence of STIs in WLHIV is of great importance as it provides information on epidemiological and social profile, in addition to sexual risk behavior. It also generates data that can be used in the implementation of prevention and treatment programs.8,12 Delay to detect precancerous lesions may have harmful consequences, mainly in HIV-infected women due to faster disease progression.¹³ Although early diagnosis of precancerous lesions can be achieved by the relatively simple Papanicolaou test, cancer is often diagnosed late.14 Additionally, Davidson et al. have reported that a significant proportion of women with invasive carcinoma had a negative cytology history before the onset of disease.15
This study aimed to determine the prevalence rates of STIs such as Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis, hepatitis B and C, and high-risk HPV (HR-HPV) in WLHIV who attended a reference center for STI/AIDS in Brazil.
MethodsStudy designA cross-sectional study was carried out in WLHIV who attended a reference center for STI/AIDS in Vitoria city, Brazil. The study prospectively enrolled 150 patients between February 2014 and October 2015 at the first appointment with a gynecologist.
Inclusion criteriaThe study included women aged 18−60 years, with a positive result for HIV infection, seen at the gynecology outpatient clinic, were not pregnant, and agreed to participate in the study.
InterviewA 20-minute, face-to-face interview was conducted using a standardized questionnaire, validated in a previous study,17 containing information on demographic (age, education, marital status, family income, place of residence); epidemiological (smoking, alcohol and drug use, condom use, number of sexual partners, sexual practices); and clinical (vaginal discharge, current and previous STIs, stage of infection with HIV, CD4 count, and viral load determination). Data was then discussed among experts for further validation, comprehensive testing for STIs, patient data, including other diagnoses and treatments. All clinical laboratory data were extracted from medical charts and entered into an electronic database. Additional data were collected through physical examination, and other health-related information, including selected family history variables and use of tobacco, alcohol, and nonprescribed (i.e., recreational) drugs.
Specimen collectionGynecological examinations for collecting cervical cytology samples, according to the Ministry of Health standards, were performed for the detection of Chlamydia trachomatis, Neisseria gonorrhoeae, and HPV. Samples were collected, transported under low-temperature conditions, and stored at -70 °C. Samples were evaluated at the Virology Laboratory, Department of Pathology, of the Federal University of Espirito Santo.
Cervical cytologyCervical cytology was performed using conventional cytology long Ayre spatula and cytobrush, collecting samples scraped from the ectocervix and endocervix. The sample was thinly smeared onto a correctly identified glass slide, which was immediately transferred to a fixative liquid (hydrated alcohol). After that, samples were sent to the Pathology Department for analysis. Slides were stained using the Papanicolaou method. According to the standards of the Brazilian Ministry of Health, analysis reports were released using Brazilian descriptive nomenclature for cytological reports.18
ColposcopyColposcopies were carried out in case of abnormalities diagnosed by cytological examinations, according to the 2011 nomenclature report colposcopy19 or when the HPV test was positive for HR-HPV. When necessary, biopsies were performed using Gaylor-medina clamps of 3 and 5 mm. The information was sent, properly identified, to the Pathology Department, and all reports were released based on the histopathological report the terminology of HPV-induced squamous epithelial lesions of the lower anogenital tract, the LAST Project.20
Testing for sexually transmitted infectionsRapid tests were used according to the manufacturer’s recommendations to screen for syphilis (Alere Syphilis), via the qualitative detection of IgG, IgM, and IgA antibodies against Treponema pallidum, characterized as a treponemal test (Importer Alere S / A, São Paulo, Brazil), hepatitis B (Vikia ® HBsAg, BioMérieux SA, France), via the qualitative detection of HBs antigen, and for hepatitis C (anti−HCV; Alere, Standard Diagnostics Inc, Republic of Korea).
Chlamydia trachomatis, Neisseria gonorrhoeae, and high- and low-risk HPV were performed through polymerase chain reaction (PCR). HPV detection by PCR was accomplished using two sets of non-degenerate primers, PGMY09/11, followed by typing using Reverse Line Blotting (RLB) and Restriction Fragment Length Polymorphism (RFLP) methodologies.21–23 Real-time PCR was performed for the diagnosis of Chlamydia trachomatis and Neisseria gonorrhoeae (COBAS 4800 CT/NG – Roche Molecular Systems, Branchburg, NJ).
Monitoring and test resultsAn appointment was scheduled for each study participant about two weeks after the day of the interview. The consultation aimed to deliver the test results and discuss treatment for any diagnosed infections. Participants also received advice on the test results and how to prevent infections, and when necessary, they underwent a colposcopy and biopsy of the cervix.
Statistical analysisAll information was coded and stored anonymously in a database created for this purpose. SPSS data entry (Statistical Package for the social sciences) version 21.0 was used. A descriptive analysis was performed, including frequency distribution for qualitative variables and average calculation and standard deviation for quantitative variables. The prevalence rates of STIs were estimated by the presence of a positive test and reported based on the frequency of the diagnosis in question and with 95% confidence intervals. Possible associations between demographic, behavioral, and clinical variables were tested by the chi-square test with Yates correction or Fisher's exact test, as appropriate.
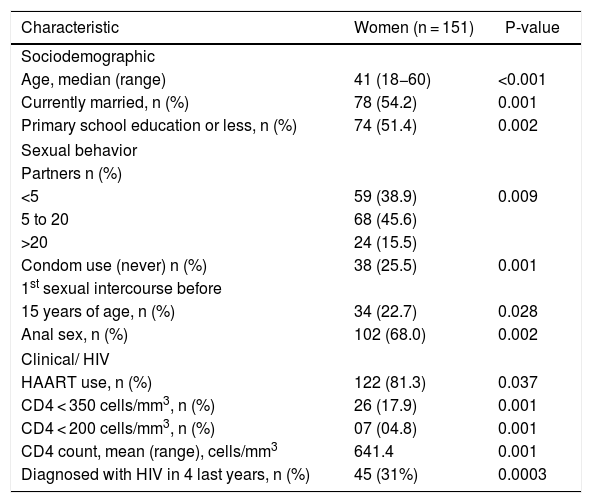
ResultsOne hundred fifty-one women receiving HIV care at the reference center for STI/AIDS were enrolled in the study between February 2014 and October 2015. Sociodemographic characteristics and risk-taking behaviors of the participants are presented in Table 1. The median age at enrollment was 41 years (SD = 10.8). Among the participants, 35.7% had been infected with HIV for 10–20 years, 5% had a CD4 count less than 200 cells/mm3, 61.6% had an undetectable HIV viral load, and 81.1% were currently on HAART; 15.4 % of women reported 21 or more lifetime sexual partners, and 22.7% reported onset of sexual activity at the age of 15 years or younger, 68% reported a history of receptive anal intercourse, 49% reported use of condoms during all sexual intercourse, and 19.3% were active cigarette smokers. In comparison, 35.6% and 24.7% had a history of alcohol ingestion or drug use, respectively (Tables 2 and 3).
Sociodemographic variables, sexual behavior, and clinical characteristics of HIV-infected woman attending an STI Clinic, Vitória, Brazil.
| Characteristic | Women (n = 151) | P-value |
|---|---|---|
| Sociodemographic | ||
| Age, median (range) | 41 (18−60) | <0.001 |
| Currently married, n (%) | 78 (54.2) | 0.001 |
| Primary school education or less, n (%) | 74 (51.4) | 0.002 |
| Sexual behavior | ||
| Partners n (%) | ||
| <5 | 59 (38.9) | 0.009 |
| 5 to 20 | 68 (45.6) | |
| >20 | 24 (15.5) | |
| Condom use (never) n (%) | 38 (25.5) | 0.001 |
| 1st sexual intercourse before | ||
| 15 years of age, n (%) | 34 (22.7) | 0.028 |
| Anal sex, n (%) | 102 (68.0) | 0.002 |
| Clinical/ HIV | ||
| HAART use, n (%) | 122 (81.3) | 0.037 |
| CD4 < 350 cells/mm3, n (%) | 26 (17.9) | 0.001 |
| CD4 < 200 cells/mm3, n (%) | 07 (04.8) | 0.001 |
| CD4 count, mean (range), cells/mm3 | 641.4 | 0.001 |
| Diagnosed with HIV in 4 last years, n (%) | 45 (31%) | 0.0003 |
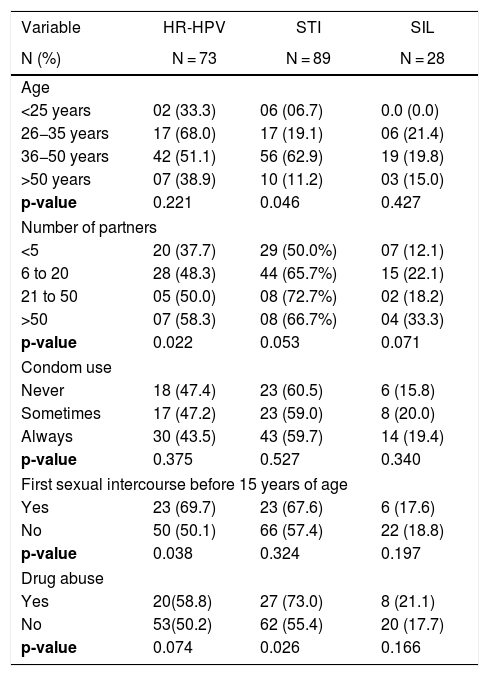
Behavioral aspects of HIV-infected patients attending an STI clinic, Vitoria, Brazil (n = 151).
| Variable | HR-HPV | STI | SIL |
|---|---|---|---|
| N (%) | N = 73 | N = 89 | N = 28 |
| Age | |||
| <25 years | 02 (33.3) | 06 (06.7) | 0.0 (0.0) |
| 26−35 years | 17 (68.0) | 17 (19.1) | 06 (21.4) |
| 36−50 years | 42 (51.1) | 56 (62.9) | 19 (19.8) |
| >50 years | 07 (38.9) | 10 (11.2) | 03 (15.0) |
| p-value | 0.221 | 0.046 | 0.427 |
| Number of partners | |||
| <5 | 20 (37.7) | 29 (50.0%) | 07 (12.1) |
| 6 to 20 | 28 (48.3) | 44 (65.7%) | 15 (22.1) |
| 21 to 50 | 05 (50.0) | 08 (72.7%) | 02 (18.2) |
| >50 | 07 (58.3) | 08 (66.7%) | 04 (33.3) |
| p-value | 0.022 | 0.053 | 0.071 |
| Condom use | |||
| Never | 18 (47.4) | 23 (60.5) | 6 (15.8) |
| Sometimes | 17 (47.2) | 23 (59.0) | 8 (20.0) |
| Always | 30 (43.5) | 43 (59.7) | 14 (19.4) |
| p-value | 0.375 | 0.527 | 0.340 |
| First sexual intercourse before 15 years of age | |||
| Yes | 23 (69.7) | 23 (67.6) | 6 (17.6) |
| No | 50 (50.1) | 66 (57.4) | 22 (18.8) |
| p-value | 0.038 | 0.324 | 0.197 |
| Drug abuse | |||
| Yes | 20(58.8) | 27 (73.0) | 8 (21.1) |
| No | 53(50.2) | 62 (55.4) | 20 (17.7) |
| p-value | 0.074 | 0.026 | 0.166 |
N: number of people within the population, X²= chi-square. HR-HPV: high-risk HPV, STI: Sexual Transmitted Infection, SIL: Squamous Intraepithelial Lesion obtained by cervical biopsy, included CIN I, CIN II, CIN III).
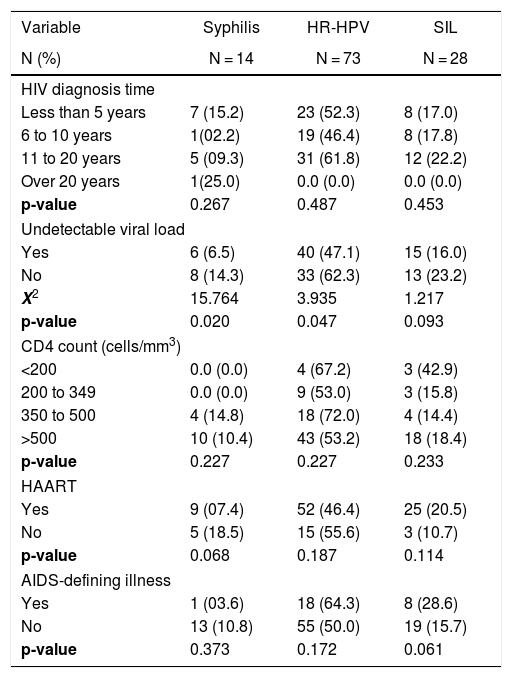
Prevalence and correlates associated with the Syphilis, HR-HPV, and SIL in HIV patients attending an STI clinic in Vitoria, Brazil (n = 151).
| Variable | Syphilis | HR-HPV | SIL |
|---|---|---|---|
| N (%) | N = 14 | N = 73 | N = 28 |
| HIV diagnosis time | |||
| Less than 5 years | 7 (15.2) | 23 (52.3) | 8 (17.0) |
| 6 to 10 years | 1(02.2) | 19 (46.4) | 8 (17.8) |
| 11 to 20 years | 5 (09.3) | 31 (61.8) | 12 (22.2) |
| Over 20 years | 1(25.0) | 0.0 (0.0) | 0.0 (0.0) |
| p-value | 0.267 | 0.487 | 0.453 |
| Undetectable viral load | |||
| Yes | 6 (6.5) | 40 (47.1) | 15 (16.0) |
| No | 8 (14.3) | 33 (62.3) | 13 (23.2) |
| X2 | 15.764 | 3.935 | 1.217 |
| p-value | 0.020 | 0.047 | 0.093 |
| CD4 count (cells/mm3) | |||
| <200 | 0.0 (0.0) | 4 (67.2) | 3 (42.9) |
| 200 to 349 | 0.0 (0.0) | 9 (53.0) | 3 (15.8) |
| 350 to 500 | 4 (14.8) | 18 (72.0) | 4 (14.4) |
| >500 | 10 (10.4) | 43 (53.2) | 18 (18.4) |
| p-value | 0.227 | 0.227 | 0.233 |
| HAART | |||
| Yes | 9 (07.4) | 52 (46.4) | 25 (20.5) |
| No | 5 (18.5) | 15 (55.6) | 3 (10.7) |
| p-value | 0.068 | 0.187 | 0.114 |
| AIDS-defining illness | |||
| Yes | 1 (03.6) | 18 (64.3) | 8 (28.6) |
| No | 13 (10.8) | 55 (50.0) | 19 (15.7) |
| p-value | 0.373 | 0.172 | 0.061 |
N: number of people within the population, X²= chi-square, HR-HPV: high-risk HPV, SIL: Squamous Intraepithelial Lesion obtained by cervical biopsy, included CIN I, CIN II and CIN III, HAART: highly active antiretroviral therapy, CD4: CD4+ T lymphocytes rate per microliter blood.
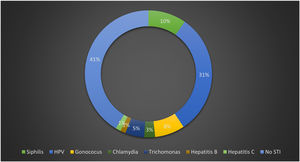
A total of 59.2% had at least one diagnosed STI at the time of the first clinic visit, with the most common being condyloma acuminatum, 31.0% had clinical forms of anogenital HPV, 10.0% had syphilis, 5.0% had trichomoniasis, 3% were infected by Chlamydia trachomatis, 8% by Neisseria gonorrhoeae, 1.0% had hepatitis B, and 1.0% hepatitis C. Fig. 1 shows the prevalence of STIs at the time of the first clinic visit.
Cervical cytological abnormalities (defined as ASC-US or higher degree of abnormality) were found in 6.7% of women, 28 (18.5%) underwent cervical biopsy and had cervical intraepithelial neoplasia (CIN), some of whom had normal cytology. Out of 28 biopsies performed, 18 (11.9%) revealed high-grade squamous intraepithelial lesion (HSIL), and 10 (6.6%) low-grade squamous intraepithelial lesion (LSIL). Overall, HR-HPV (HPV 16, 51, 52, 53, 45, 18, 39, 59, 58, 33, 31, 68 and 35) types were present in 73 (48.3%) patients.
DiscussionGlobally, the burden of HPV infection, causing cervical neoplasia, is high in developing countries. The oncogenic nature of the genotypes differs in each region of the world. A Brazilian study published in 2013 identified that 170 of 172 (99%) women with cancer were found to be positive for HPV.28 The most frequent HPV genotypes observed were HPV16 (77.6%), HPV18 (12.3%), HPV35 (5.9%), HPV31 (8.8%), and HPV33 (7.1%), most often (75%) in isolation.29 The most frequent HR-HPV In our study 16 (13.5%), 51 (9.5%), 52 and 53 (both 5.6%) were the prevailing genotypes.
Overall, studies showed that the prevalence of HPV infection in WLHIV in Brazil is high, whereas that of CIN is low. These findings may be due to the vast majority of studies being conducted in regions that are more developed, where HIV-infected women have better follow-up and use HAART more correctly, thus with less risk to development cervical lesions.30 WLHIV were 4.5 times more likely than uninfected women to develop cervical intraepithelial neoplasia.24,25 The rate of high-risk HPV in our study was quite high, 48.3% (73/151). This rate was even higher in women who had sexual intercourse for the first time before age 15 (69.7%) and in those who had more than 50 partners throughout their life (58.6%). A cohort study in Denmark found an HPV rate of 28% among HIV-infected women.26
The prevalence of CIN lesions (18.5%) among WLHIV in this study was very similar to that reported in a study in HIV-negative women at a colposcopy clinic in Brazil (18.2%).14 However, the prevalence of CIN among women who reported more than 50 partners over their lifetime in our study was significantly higher (33%), and even higher than women with CD4 < 200/mm³ (42%), although HIV-associated immunosuppression is known to alter the relative carcinogenicity of HR-HPV types.27
A high prevalence of prior STIs (59.2%) was found in this study at a rate similar to that found in a study in the city of São Paulo, Brazil, in which the prevalence of was STIs around 61% in a group of women living with AIDS.12 Among drug users, the prevalence of STIs reached 73.0%. In the present study, the prevalence of STIs was also exceedingly high in the age group between 35 and 50 years (69.0%) and among women with more than 20 partners (72.7%). Although several low-income countries have achieved WHO targets for eliminating congenital syphilis, increased rates of this STI in HIV-infected people is a strong reminder of the tenacity of Treponema pallidum infection.31 The prevalence of syphilis was higher in women who had not received HAART (18.5%) and among women with detectable viral load (14.3%). In a cohort study of 2410 patients in Switzerland, from 1995 to 1997, the incidence of opportunistic infections decreased from 15.1 per 100 person-years in the six months before initiating HAART to 7.7 and 2.6 per 100 person-years in the first three and six months after starting HAART, respectively.32
The many limitations and challenges of implementing treatment as prevention should be pointed out to emphasize that sustained, population-level prevention benefit from earlier and wider use of HAART. It is important to highlight that HAART does not prevent other STIs and can give a false idea of complete protection.
ConclusionA high prevalence of STIs was observed among HIV-infected women in our investigation. Most patients were on HAART and only 5% of them had a CD4 count <200 cells/mm3. However, more than half of the women were diagnosed with at least one STI at the first medical visit and reported unprotected sex.
Prevention programs need to focus on counseling for WLHIV and their regular partners with interventions such as couple counseling and education programs. Improved cervical cancer screening is urgent to prevent the expected increase in invasive cervical cancer among HIV-infected women with improved life expectancy as a result of HAART.
Ethical aspectsThis project was submitted to the Research Ethics Committee of the Health Sciences Center at the Federal University of Espírito Santo and requested a letter of consent from the committees of the other participating centers. All information reported by participants was only used for research purposes, and data confidentiality was always assured. In several ways, privacy was protected: all interviewers were trained for HIV testing counseling and had experience in maintaining confidentiality.