We describe three cases of community-acquired necrotizing pneumonia which were caused by Panton–Valentine leucocidin-producing strains of Staphylococcus aureus (one of them methicillin sensitive). All cases were successfully treated without any sequelae for the patients due to the prompt initiation of adequate antimicrobial therapy. High suspicion toward this fatal pathogen was the key to the successful outcome of the patients.
Community-acquired pneumonia due to Staphylococcus aureus is an infrequent but potentially lethal infection.1,2 It is often associated with the production of Panton–Valentine leucocidin (PVL), which is responsible for extensive tissue necrosis and a high mortality rate.3 Herein we present three cases of necrotizing pneumonia, one of them caused by a methicillin susceptible strain, all of which had a favorable outcome.
Case historiesCase 1A previously healthy 18-year-old boy, a Panama resident traveling in Europe, was admitted to the ICU of our hospital suffering from pneumonia and severe respiratory distress. He was transferred from another hospital where he presented with high fever, weakness and productive cough. He had been hospitalized for three days and received oseltamivir, ceftriaxone and moxifloxacin. He reported having a flu-like syndrome some days earlier.
At ICU admission he had a temperature of 38°C, a respiratory rate of 40 per min, a heart rate of 120beats/min and he was hemodynamically stable. Thorax auscultation revealed crackles over the lower and middle, right lung fields, while chest X-ray (CXR) showed patchy alveolar opacities in the right lung. Blood gases on admission showed: pH 7.31, pCO2: 50mmHg, pO2: 65mmHg in a non-rebreathing mask. Laboratory findings revealed a white blood count (WBC) of 1220cells/L (59% neutrophils) and a CRP of 306mg/L. The Acute Physiology and Chronic Health Evaluation (APACHE) II severity score was 21. His respiratory distress gradually increased and 24h later he was intubated and needed inotropic support. The new CXR revealed that alveolar type infiltrates were spread in both lungs. Urine antigens for Legionella pneumophila type 1 and Streptococcus pneumoniae were negative.
A bronchoscopy was performed and the Gram stain of the BAL revealed the presence of numerous Gram-positive cocci. The treatment was switched to linezolid and moxifloxacin. Cultures from BAL specimens yielded a PVL, methicillin sensitive S. aureus (MSSA) while blood cultures were negative. Test for HIV was negative.
Hydrocortisone was added to the therapeutic regimen because of the septic shock and filgrastim was given for the leukopenia. Clindamycin was also added in order to diminish the toxin production by the PVL strain.4
On day 8 the fever subsided but his course was complicated by a spontaneous pneumothorax, which necessitated the insertion of a pleural catheter. Subsequently he suffered an episode of bacteremic ventilator-associated pneumonia by a carbapenem-resistant Klebsiella pneumoniae, which was successfully treated with colistin and tigecycline. The patient was extubated on day 21 and he was finally discharged on day 27.
Case 2A 34-year-old woman was transferred to our ICU from the Department of Obstetrics and Gynecology due to severe respiratory failure six days after in vitro fertilization. She had no past medical history. The embryo-transfer was complicated by Ovarian Hyperstimulation Syndrome. On admission the APACHE II score was 26 and blood gases values were pH: 7.33, pO2: 80mmHg, pCO2: 38mmHg on facemask providing FiO2 of 40%. Auscultation revealed diffuse crackles. During the next 6h her temperature rose to 39.5°C and she presented productive cough with dark sputum and shortness of breath at rest, so she was admitted to the ICU. CXR on ICU admission revealed an infiltrate of the left middle lung field and bilateral pleural effusions. Laboratory tests showed a WBC of 3700/L, CRP of 298mg/L, procalcitonin of 36ng/mL, fibrinogen of 507mg/dL, and hypogammaglobulinaemia, while human chorionic gonadotropin (hCG) levels were compatible with pregnancy. Sputum Gram stain revealed Gram-positive cocci and urinary antigens for L. pneumophila type 1, and S. pneumoniae and pharyngeal aspirate for Influenzae A and B were negative. Treatment with intravenous linezolid and clindamycin was immediately started because the severity of the patient's condition and the isolation of Gram-positive cocci in the sputum supported the clinical suspicion of community-acquired staphylococcal pneumonia. Nevertheless, the patient worsened and became hemodynamically unstable requiring intubation and vasopressors. She was also given hydrocortisone and activated protein C. The blood cultures and the tracheal aspirate grew MRSA. The patient showed gradual hemodynamic improvement and was weaned from vasopressors after three days, while hCG values declined rapidly returning to nonpregnant range. A chest CT scan was performed and showed bilateral infiltrates with multiple cavitating lesions, left lung abscess formation and bilateral pleural effusions. Culture of pleural fluid developed also MRSA. Her course was complicated by nosocomial bacteremia and she was submitted to a tracheostomy. Gas exchange as well as lung mechanics gradually stabilized and she was finally weaned from mechanical ventilation. She was discharged to the ward on day 37 and she had a long rehabilitation period.
Case 3A 33-year-old male with no significant past history was admitted to the ICU of our hospital because of acute respiratory failure due to a lower respiratory tract infection. He presented to the Emergency Department several days earlier complaining for chest pain, high temperature (39°C) and progressive dyspnea with blood-stained sputum. The CXR revealed the presence of bilateral pulmonary infiltrates. After his admission to the internal medicine department his situation progressively deteriorated and two days later he was intubated and transferred to the ICU. His treatment initially included ceftriaxone and clarithromycin but because of the severity of his situation a staphylococcal pneumonia was suspected and the antibiotic regimen was empirically modified to linezolid and moxifloxacin. On ICU admission he presented hemodynamic instability and had to be resuscitated with intravenous fluids and norepinephrine. Initial laboratory data obtained in the ICU showed WBC: 17.890/L (neutrophils: 82%), CRP: 250mg/L. Arterial blood gases analysis showed respiratory acidosis and severe hypoxemia (pH: 7.11, pCO2: 91mmHg, pO2: 56mmHg – in assist control ventilation, FiO2: 100%). Blood cultures and sputum specimens taken in the ward grew MRSA. The blood cultures remained positive after seven days of treatment with linezolid and daptomycin. Clindamycin were added to the regimen. Two transesophageal echos performed in fifteen days time did not reveal the presence of vegetations. Of note, HIV and tests for other respiratory pathogens were negative.
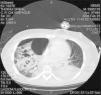
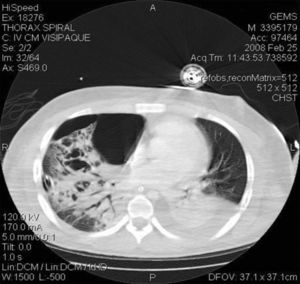
The patient's course was complicated by the development of spontaneous pneumothorax and empyema of the right lung (Fig. 1). He had to be submitted to lung decortication. MRSA was also isolated from the culture of the pleural fluid. On day 23 he was submitted to a tracheostomy. His situation ameliorated, the tracheostomy was closed and he was transferred to the ward on day 40. He was discharged from the hospital one month later. It should be noted that one of the herein described cases has been previously published.5
In vitro evaluation of S. aureus clinical isolatesSeven isolates were available for further testing. Susceptibilities to various antimicrobial agents were evaluated by an automated system (BD Phoenix automated microbiology system; BD Diagnostic Systems, Sparks, MD) (Table 1). All isolates were found to carry the lukF-PV and lukS genes coding for Panton–Valentine leukocidin (PVL),6 while all isolates of cases 2 and 3 were found to also carry the mecA gene in type IVc/e staphylococcal cassette chromosome mec element (SCCmec).7
Minimum inhibitory concentrations (MICs) of 15 antimicrobials against S. aureus study isolates.
| Case | Isolate no | Source | P | OX | FOX | VA | TEC | GM | E | CIP | LVX | CC | SXT | RA | SYN | LZD | FA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m 4442 B | BAL | >1 | 0.5 | ≤2 | ≤1 | ≤1 | ≤2 | 0.5 | ≤0.5 | ≤1 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | 2 | ≤2 |
| 2 | SP 8728 | Sputum | >1 | >2 | >8 | ≤1 | ≤1 | ≤2 | >4 | ≤0.5 | ≤1 | >2 | ≤0.5 | ≤0.5 | ≤0.5 | 2 | 4 |
| SP 8753 | Tracheal aspirate | >1 | >2 | >8 | ≤1 | ≤1 | ≤2 | >4 | ≤0.5 | ≤1 | >2 | ≤0.5 | ≤0.5 | 1 | 2 | 8 | |
| P 6538 | Pleural-fluid | >1 | >2 | >8 | ≤1 | ≤1 | ≤2 | >4 | ≤0.5 | ≤1 | >2 | ≤0.5 | ≤0.5 | 1 | 2 | 8 | |
| b 8503 | Blood | >1 | >2 | >8 | ≤1 | ≤1 | ≤2 | >4 | ≤0.5 | ≤1 | >2 | ≤0.5 | ≤0.5 | 1 | 2 | >8 | |
| 3 | b 3286 | Blood | 1 | 2 | 8 | ≤1 | ≤1 | ≤2 | ≤0.25 | ≤0.5 | ≤1 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | 2 | 4 |
| m 2332 | Pleural-fluid | 1 | 1 | 4 | ≤1 | ≤1 | ≤2 | ≤0.25 | ≤0.5 | ≤1 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 | 2 | 4 | |
The PFGE patterns of SmaI-digested DNA of all isolates of cases 2 and 3 were identical. MLST typing performed only to blood isolates linked PFGE pattern with the well-described ST80 clone that seems to be spreading through Europe. Isolate m4442B of case 1 was lost to subculture and was not submitted to MLST typing.
DiscussionS. aureus is a commonly recognized cause of infections in humans. These infections can become life-threatening due to the ability of the microorganism to produce virulence factors including adhesion factors and toxins. PVL is a toxin produced by S. aureus strains (carrying the lukS-PV and lukF-PV genes) often associated to severe skin and soft tissue infections but also causing necrotizing pneumonia with a high mortality. The presence of this toxin has been detected among MRSA as well as MSSA strains.
The usual patient with PVL-associated S. aureus pneumonia is a child or a young otherwise healthy adult. A viral illness can precede pneumonia while leucopenia attributed to the toxin is not an uncommon finding. A specific disease entity has also been ascribed to PVL-positive S. aureus-associated respiratory infection including a rapidly progressing hemorrhagic and necrotic pneumonia which can cause Acute Respiratory Distress Syndrome (ARDS) possibly leading to septic shock and multi-organ failure.
We describe three patients with severe pneumonia caused by Panton–Valentine-producing S. aureus. In two cases bacteremia was also present. Diagnosis of influenza was not confirmed in any case while prodrome flu-like symptoms were present in only one patient. Both MRSA isolates belonged to ST80, which is the most common clone causing infection in the community as well as in the hospital in Greece.8 Among bloodstream infections caused by MRSA, the hospital-associated clone ST239 and sporadic cases of ST30, ST97 and ST3989 follow ST80. One of the study isolates was MSSA producing PVL. Although rare, the production of PVL toxin by MSSA strains has been reported previously.6
Three similar cases due to MRSA strains have also been described in Greece, two of them with an unfavorable outcome.10,11 The mortality of PVL-positive CAP lies between 45% and 56%.7,8 The severity of the disease is similar whether the isolate is a methicillin-resistant strain or not because it is associated to other factors such as multi-organ failure, ICU admission, mechanical ventilation, leukopenia, necrotizing pneumonia, shock, disseminated intravascular coagulation, rash, ARDS, airway bleeding and development of complications.12,13
Our patients presented some of these factors such as ICU treatment, mechanical ventilation due to pneumonia-induced ARDS and need for inotropic support while all of them developed several complications. Two cases had leucopenia on admission, which is considered to be inversely associated with survival. On the contrary, another unfavorable prognostic factor, airway bleeding, was absent possibly contributing to the favorable outcome.
The optimal treatment of this often lethal disease has not been established. Since the detrimental effects of the infection are mainly caused by the production of PVL rather than the microorganism itself, an effective treatment should target first at the eradication of S. aureus but additionally at diminishing the effects of the toxin. Drainage of the suppurative collection, if possible, is suggested in order to remove PVL containing tissues. Furthermore certain antimicrobial agents are able to reduce the production of PVL. Such molecules include clindamycin, linezolid and rifampicin.14 These antimicrobial agents are effective in reducing PVL production even at suboptimal concentrations in necrotic tissues. Dumitresku et al. have shown that if other agents such as beta-lactams are used and levels above the MIC (minimum inhibitory concentrations) are not achieved in the infected tissues, PVL secretion may be enhanced.14 Moreover upregulation of PVL toxin observed with nafcillin15 could also result with flucloxacillin when used for treating MSSA.16 As for clindamycin additional advantages of the drug include its ability (a) to decrease Toxic Shock Syndrome Toxin-1 production17 and (b) to stop production of alpha-toxin by translational inhibition.18 Combined use of clindamycin and linezolid has been described as having successful results on PVL toxin and patients’ survival in two of three cases of necrotizing pneumonia.19 On the other hand vancomycin, a traditional drug for the treatment of MRSA, has no impact on exotoxin formation.20 Polyvalent human intravenous immunoglobulin (IVIg) is an adjunctive therapy for serious PVL-associated infections. It acts by neutralizing the PVL pore formation and by inhibiting the cytotoxic effects of PVL on polymorphonuclear cells as shown by Gauduchon et al.21 Although the result appears to be dose-dependent, the usually reported dose does not exceed 2g/kg.16 The use of IVIg has been reported to have a benefit on patients’ survival in sporadic cases.19,22,23
All our patients received a combination of linezolid and clindamycin agents that provide both antimicrobial action and anti-toxin effect. In the second case we used IVIg as adjunctive therapy, a factor probably contributing to patients recovery. Of note, although the use of linezolid is only recommended for hospital-acquired MRSA pneumonia, it has the advantage of achieving high concentrations in the epithelial lining fluid of the lung24 and it is a reasonable first choice for community-acquired MRSA pneumonia as well, particularly in the suspicion of PVL for the aforementioned reasons.
Panton–Valentine associated pneumonia remains a disease entity with a high fatality rate. A high degree of awareness is necessary in order to initiate a proper and aggressive treatment even in the absence of a preceding flu-like syndrome. As the identification of the toxin is not always feasible or quickly performed, empirical choice of antimicrobials is of paramount importance.
FundingNo grant or financial support was received for the study.
Conflicts of interestThe authors declare no conflicts of interest.