There are no specific recommendations for prevention of surgical site infection (SSI) caused by multidrug resistant Gram-negative bacilli (MDR-GNB). Our objective was to systematically review the literature evaluating the efficacy and safety of measures specifically designed to prevent MDR-GNB SSI.
MethodsWe searched MEDLINE, EMBASE, CINAHL and LILACS databases up to February 18, 2020. Randomized trials and observational cohort studies evaluating the efficacy of preventive measures against MDR-GNB SSI in adult surgical patients were eligible. We evaluated methodological quality of studies and general quality of evidence using Newcastle-Ottawa scale, Cochrane ROBINS-I and GRADE method. Random-effects meta-analyses were performed using Review Manager V.5.3 software.
ResultsA total of 10,663 titles by searching databases were identified. Two retrospective observational studies, comparing surgical antibiotic prophylaxis (SAP) with or without aminoglycoside in renal transplantation recipients, and one non-randomized prospective study, evaluating ertapenem vs. cephalosporin plus metronidazole for SAP in extended spectrum beta-lactamase producing Enterobacteriales carriers undergoing colon surgery, were included. Risk of bias was high in all studies. Meta-analysis was performed for the renal transplantation studies, with 854 patients included. Combined relative risk (RR) for MDR GNB SSI was 0.57 (95%CI: 0.25-1.34), favoring SAP with aminoglycoside (GRADE: moderate).
ConclusionsThere are no sufficient data supporting specific measures against MDR-GNB SSI. Prospective, randomized studies are necessary to assess the efficacy and safety of SAP with aminoglycoside for MDR-GNB SSI prevention among renal transplantation recipients and other populations. PROSPERO 2018 CRD42018100845.
Surgical site infection (SSI) occurs in 3% to 20% of procedures, with relevant morbidity and mortality.1 When multidrug resistant (MDR) bacteria are involved, outcomes are worse, with higher cost and longer hospital stay.2,3 Among MDR microorganisms, Gram-negative bacilli (GNB), such as extended spectrum beta-lactamase (ESBL) producing and carbapenem resistant GNB, are of great importance due to the diversity of resistance mechanisms, dissemination capacity, high morbidity, and scarcity of effective treatment.4,5
Increasing concerns regarding MDR GNB SSI6,7 led to studies estimating incidence rates, expressed as percentage of all surgeries under surveillance that developed MDR GNB SSI, as high as 5.2% of general surgery in Ghana and 3.2% of major hepatectomy in Japan.8,9
Surgical antibiotic prophylaxis (SAP) adjustment and decolonization is well established for patients colonized by methicillin resistant Staphylococcus aureus (MRSA).10 However, there is no specific recommendation regarding SAP adjustment or other preventive measures against MDR GNB SSI.7,10
The objective of this study is to systematically review the literature to evaluate the efficacy and safety of measures to prevent SSI caused by MDR GNB.
Materials and methodsWe searched MEDLINE (PubMed), EMBASE, CINAHL and LILACS databases, without language or date restrictions, up to February 18th, 2020. Studies that met the following elements were included: (a) Population - adult patients at risk of MDR GNB SSI; (b) Intervention - SAP containing antimicrobials with action on MDR GNB (Appendix - Table A1) or other measures specifically aimed at preventing MDR GNB SSI; (c) Comparator - standard SAP, according to current recommendations for the surgery in question (10); (d) Outcome (main): SSI caused by MDR GNB; secondary outcomes: total incidence of SSI, incidence of any healthcare associated infections (HAI), length of hospital stay, adverse events, and hospital mortality from any cause; (e) Study design - intervention studies, whether randomized or not, and observational cohort studies (see full search strategy on Appendix - Text A1).
Exclusion criteria were: a) unreported incidence of SSI stratified by etiology and antimicrobial resistance status; b) absence of MDR definition; c) two or more intervention measures implemented concomitantly, making it impossible to tease out their individual effects on outcome; d) report of different SSI standard preventive measures (e.g., surgical antisepsis protocol) between intervention and comparator groups.
Four groups of two authors independently reviewed abstracts and titles using Rayyan QCRI tool (Quatar Computing Research Institute), selected full texts and their references, and extracted data using a standardized form on REDCap software.11 Data extracted comprised authors, country, and date of study; type and characteristics of intervention and comparator; type of surgery; sex and age of participants; study design; outcome data; funding sources. Two authors independently evaluated the methodological quality of studies using the Newcastle-Ottawa (NO) scale for cohort studies12 and Cochrane ROBINS-I, and assessed the general quality of the evidence and strength of the recommendation with the GRADE method.13 Disagreements were resolved by discussion or, if consensus was not achieved, referred for a third reviewer. For studies with comparable populations and interventions, random-effects meta-analyses were performed using Review Manager Version 5.3. Ethics approval was not required as the research was a systematic review. This review's protocol was registered at PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018100845).
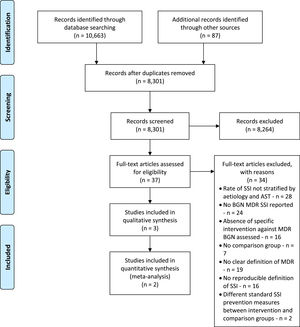
ResultsA total of 10.663 titles by searching databases were identified, and 87 additional titles from references of studies selected for full-text evaluation. After removal of replicates, 8,301 abstracts remained for evaluation, of which 39 were selected for full-text evaluation (Fig. 1). Only one study met the criteria for inclusion14. Multiple emails were sent for different authors requesting supplementary information. After the authors' reply, two additional articles met the eligibility criteria15,16.
In the multicenter, prospective, open and non-randomized study published by Nutman et al.,14 the population consisted of adult patients colonized with ESBL-producing Enterobacteriales (ESBL-PE) preoperatively, who underwent elective colorectal surgery in three hospitals in Israel, Serbia and Switzerland, between 2012 and 2017 (Table 1).
Characteristics of the eligible studies.
| First author, year | Abboud, 2013 | Freire, 2015 | Nutman, 2019a |
|---|---|---|---|
| Country | Brazil | Brazil | Israel, Serbia and Switzerland |
| Study design | Observational retrospective | Observational retrospective | Non randomized interventional prospective |
| Type of surgery | Renal transplantation | Renal transplantation | Colon surgery |
| Antibiotic prophylaxis regimen – (N) | |||
| Intervention | Cefuroxime + Gentamicin (21) | Amikacin (233) | Ertapenem (269) |
| Comparison | Cefuroxime (24) | Cephalosporin (576) | Cephalosporin + Metronidazol (209) |
| Surgical Site infection caused by MDR GNB – n (%) | |||
| Intervention | 0 (0.0%) | 6 (2.6%) | 4 (1.5%) |
| Comparison | 3 (12.5%) | 23 (4.0%) | 15 (7.2%) |
| Relative Risk (RR) (95%CI) | 0.16 (0.01-2.97) | 0.64 (0.27-1.56) | 0.21 (0.07–0.62)b |
| Surgical Site infection-any cause - n (%) | |||
| Intervention | 2 (9.5%) | 17 (7.3%) | 47 (17.5%) |
| Comparison | 4 (16.7%) | 89 (15.5%) | 45 (21.5%) |
| RR (95%CI) | 0.57 (0.12-2.77) | 0.47 (0.29-0.76) | 0.81 (0.56-1.17) |
| Healthcare associated infection (HAI) - n (%) | |||
| Intervention | 4 (19%) | NR | NR |
| Comparison | 13 (54%) | NR | NR |
| RR (95%CI) | 0.35 (0.15-0.83) | - | - |
| Length of hospital stay | NR | NR | NR |
| Adverse eventsc- n (%) | |||
| Intervention | NR | 85 (36.5%) | NR |
| Comparison | NR | 169 (29.2%) | NR |
| RR (95%CI) | - | 1.24 (1.00-1.54) | - |
| Death in 30 daysd- n (%) | |||
| Intervention | 1 (4.8%) | NR | NR |
| Comparison | 2 (8.3%) | NR | NR |
| RR (95%CI) | 0.57 (0.06-5.79) | - | - |
GNB, Gram Negative Bacilli; MDR, Multidrug resistant; n, number of events in each group; N, number of subjects in each group; NR, not reported.
In the baseline phase (BP), routine SAP consisted of cefuroxime plus metronidazole. In the intervention phase (IP), for patients colonized with ESBL-PE, ertapenem replaced routine SAP. The primary outcome was any SSI, and secondary outcomes were deep or organ/space SSI and ESBL-PE culture-positive SSI.
Of the 3,600 patients screened preoperatively, 498 had ESBL-PE colonization, of whom 478 were included in the intention to treat (ITT) analysis: 209 in the BP and 269 in the IP.
In the ITT analysis, 45 (21.5%) patients in the BP developed SSI, compared with 47 (17.5%) in the IP, which was not significantly different. Deep SSI rates did not differ significantly as well. The ESBL-PE culture-positive SSI rate was significantly lower in the IP compared to the BP (Table 1).
There were no significant differences regarding postoperative adverse events, including length of stay in the intensive care unit (ICU), acute kidney injury (AKI), recurrent surgery, Clostridioides difficile infection or death. It is noteworthy that these results were reported only for the as-treat analysis, in which participants who actually received the routine prophylaxis (n = 247) were compared to those who received ertapenem (n = 221).
In the study by Freire et al.,16 the population consisted of patients undergoing kidney transplantation from January 2009 to December 2012 at the Clinical Hospital of the University of São Paulo, Brazil (Table 1).
From January 2009 to July 2012, the standard SAP was cefazolin administered for three days. In addition, between January 2009 and December 2010, surgeons could change prophylaxis to ceftriaxone or amikacin. Between August 2012 and December 2012, the standard SAP was amikacin. Patients using cephalosporin (n = 576) and amikacin (n = 233) were compared (Table 1).
The main outcome was SSI during the first 60 days after transplantation. Secondary outcomes were SSI by MDR, implanted graft survival, delayed implanted graft function (DGF) and death after one year of transplantation. Incidence rates of SSI and SSI by MDR were expressed as the number of events per 1,000 patients/day. Data on the outcome of MDR GNB SSI expressed as a proportion (cumulative incidence) were supplemented by contact with the authors. Of note, the use of amikacin as antibiotic prophylaxis was associated with significantly less SSI in the multivariate analysis (odds ratio [95%CI] 0.37 [0.15–0.92]).16
In the study by Abboud et al.,15 the population consisted of patients undergoing kidney transplantation at the Dante Pazzanese Institute of Cardiology, São Paulo, Brazil. The control group was selected from a historical cohort from September 2009 to June 2010 and consisted of 24 patients who received SAP with cefuroxime (Table 1). Between July 2010 and April 2011, gentamicin was added to the SAP regimen, along with strengthening infection control measures, which was motivated by the observation of an increase in HAI caused by GNB, including carbapenem resistant K. pneumoniae, in renal transplantation patients. Despite reporting that an average of 40 kidney transplants were performed per year, the total number of patients who underwent kidney transplantation during the study period was not described, nor were the criteria for selecting the patients who formed both control and intervention groups. The main outcome was the 30-day HAI rate after kidney transplantation. Other outcomes evaluated were SSI, death within 30 days postoperatively, urinary tract infection (UTI), primary bloodstream infection, and pneumonia. Data on the outcome of MDR GNB SSI were supplemented by contact with the authors.15
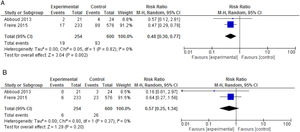
We carried out a meta-analysis of data from Abboud et al.15 and Freire et al.;16 854 patients were included, with a mean follow-up of 45 days (Fig. 2). The combined relative risk (RR) for MDR GNB SSI was 0.57 (95% CI: 0.25-1.34), with heterogeneity test I2 = 0% (p = 0.37). For the total SSI outcome, the combined RR (95% CI) was 0.48 (0.30-0.77), with I2 = 0% (p = 0.82), thus favoring intervention (GRADE: high). Abboud et al.(15) also reported a lower incidence of HAI in the intervention group (54.2% (13/24) vs. 19% (4/12), p = 0.03). Freire et al.16 reported a greater, yet not statistically significant, incidence of DGF in the amikacin group, with an RR (95% CI) = 1.25 (1.01-1.54); on the other hand, loss of renal graft was less frequent among these patients, again without statistical significance. Abboud et al.15 mentioned that patients who used gentamicin did not exhibit worsening renal function, but data were not reported.
None of the three studies addressed the impact of intervention on length of hospital stay and on antimicrobial resistance.
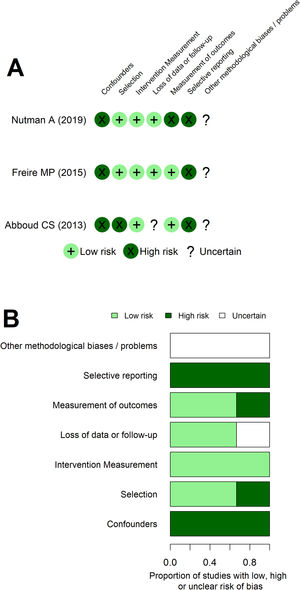
The risk of bias assessment with the NO scale (Appendix - Table A2) and ROBINS tool (Fig. 3) returned similar interpretations. Both assessments showed Abboud's work as more predisposed to bias. Additionally, the dimension regarding the confounders/comparability between cohorts assessment was the main issue increasing the risk of bias of all studies. At ROBINS’ tool, an issue regarding selective reporting was apparent. These interpretations coincided with the understanding of methodological weakness of non-randomized intervention studies and with the review primary objective being slightly different from the original studies, requiring additional data from the authors. Additionally, GRADE assessment showed high certainty of benefit for some outcomes, such as MDR GNB SSI after colorectal surgery and SSI in renal transplantation patients. However, most outcomes have very low to moderate certainty of benefit at GRADE assessment (Appendix - Table A3).
DiscussionMost guidelines about SSI prevention do not recommend specific measures against MDR GNB.17,18 Despite increasing concerns about MDR GNB SSI, the evidence on this topic is very scarce, as highlighted by the inclusion of only three studies in this review. Another issue regarding the scarcity of evidence is when to recommend prophylaxis against MDR GNB: according to MDR GNB colonization status14 versus universal anti-MDR GNB SAP in high incidence MDR GNB endemic/epidemic settings.15,16
Colonization by MDR microorganisms increases the risk of infections caused by these pathogens,19 and for patients with known or at high risk for MRSA nasal carriage (e.g., history of MRSA colonization or infection, or a very high prevalence of SSI caused by MRSA at the hospital), especially those undergoing high-risk surgery (cardiac, orthopedic), a glycopeptide plus a beta-lactam is recommended as prophylaxis, accompanied by other measures for decolonization.10,20 On the other hand, the role of MDR GNB colonization on SSI risk is not well established, although a higher incidence of SSI caused by ESBL-PE has been already demonstrated in ESBL-PE colonized adult patients who underwent colorectal surgery and in children who underwent cardiac surgery.20-22 Current SAP guidelines suggest to adapt SAP according to local needs, but do not recommend MDR GNB screening before surgery nor establish MDR SSI prevalence thresholds over which empirical SAP adaptations would be recommended.10,20 Recently, the Spanish Guideline on SAP commented about targeted SAP for colonized ESBL-PE patients, stating that it should only be considered in "high-risk patients" (Level of evidence BIII), even though the term "high risk patients'' was not further clarified.20 World Health Organization (WHO) guidelines for SSI prevention highlighted the urgent need of well-designed studies to address these issues.18
A systematic review that included 12,350 patients submitted to transrectal ultrasound-guided prostate biopsy (TRUSPB) evaluated the effectiveness of targeted antibiotic prophylaxis (TAP) for fluoroquinolone (FQ)-resistant Enterobacteriales colonized patients, versus empiric antibiotic prophylaxis (EAP) in preventing infection.23 Infectious complication incidence was 3.4% in EAP and 0.8% in TAP patients. TRUSPB is not a surgical procedure, but these results raise the issue of the need to adapt the SAP according to the patient MDR GNB colonization profile, especially in clean contaminated or contaminated surgeries involving gastrointestinal or urogenital tracts, where Enterobacteriaceae are highly likely to be involved in surgical wound colonization and infection.20
Nutman et al. showed a significant reduction of ESBL-PE SSI incidence among ESBL-PE colonized colorectal surgery patients who underwent targeted SAP with ertapenem, compared to standard SAP patients (cephalosporin plus metronidazole), with no short-term adverse events differences between groups.14
Transplant patients have a high disease burden due to MDR GNB.24 However, experts disagree on adjusting SAP for patients undergoing solid organ transplantation according to MDR GNB gut colonization status.24,25 In 2018, a Spanish group of specialists recommended that patients colonized with ESBL-PE should receive a targeted prophylaxis regimen, however avoiding carbapenem whenever possible (BIII). The same committee recommended against targeted prophylaxis among carbapenase-producing Enterobacteriales, unless in centers with a high incidence of SSI caused by those agents (BIII)24. On the other hand, the guidelines from the American Society of Transplantation Infectious Diseases Community of Practice states that "the role of targeted ESBL-PE perioperative prophylaxis remains undefined".25
The results presented here, although questionable due to high risk of bias, show a significant effect of universal SAP with aminoglycoside in preventing SSI, compared to SAP with cephalosporin alone, in a population of patients submitted to renal transplantation in settings with high incidence of MDR GNB infections. This effect is at least partially due to the reduction in MDR GNB SSI infections, even though the small number of events make it difficult to interpret the observed effect.
Nephrotoxicity associated to SAP with aminoglycoside was not clinically relevant in this population, maybe because in both studies the duration of prophylaxis was short (one dose before incision, and a second one 24 hours later), and administered once a day.26 In previous SAP regimens, when substantial nephrotoxicity occurred, aminoglycoside was administered twice or three times a day for three to five days.27
It is worthy to interpret these findings with caution. AKI is a frequent complication after major surgery, affecting approximately 10% of patients, and it is associated with increased morbidity and mortality.28 Mean postoperative serum creatinine levels are higher among patients who receive SAP with gentamicin, compared to SAP with amikacin. However, AKI seems to be transient, and serum creatinine levels tend to return to normal after 30 days.29
This scarce evidence of benefit of broad-spectrum SAP is followed by concerns regarding emergence of resistance.30 This is especially true for carbapenems, as these antibiotics are recognized as increasing the risk for carbapenem-resistant GNB infections.31 SAP remarkably contributes to total antibiotic consumption in healthcare facilities, and might correlate to overall increased antibiotic resistance and hospital healthcare costs.32
The studies included in this systematic review addressed neither the impact of antibiotics used as SAP on the incidence of MDR GNB colonization and infection on hospital level and over time, nor the cost-effectiveness of the described interventions, thus limiting safety and cost-effectiveness assessment.
The results of this systematic review should be cautiously interpreted, due to several methodological aspects ultimately seen as high risk of bias. In addition, given the before-after study design of the three included studies, it is not possible to exclude the potential confounding of other preventive measures implemented simultaneously, especially in the study by Abboud et al, that, even though does not describe new interventions besides changing SAP, recognize the strengthening of infection control measures in a scenario of increased HAI.
In conclusion, there is some low-quality evidence supporting that SAP against MDR GNB, with either aminoglycosides or ertapenem, may prevent MDR GNB SSI after renal transplantation and colon surgery in adults, respectively, in settings with high incidence of MDR GNB SSI and/or in ESBL-PE colonized patients. Risk of nephrotoxicity with aminoglycosides was low; impact on antimicrobial resistance was not assessed and is of great concern. Prospective, randomized studies, in different surgical populations, including children, may further clarify issues regarding confounding effects, choosing the high-risk patients or a prevalence threshold for targeted prevention; elucidate medium and long-term effect on MDR emergence; and better establish the cost-effectiveness and safety of SAP and other measures against MDR GNB SSI.
This research was financially supported by the Brazilian Ministry of Health and the Pan American Health Organization/World Health Organization (PAHO / WHO) (Grant number: SCON2018-00408).