To evaluate the effectiveness of Topical Oxygen Jet Therapy (TOJT) in the treatment of surgical wounds in adult patients who has clinical signs of infection for over 30 days; and to identify the pathogens causing complicated skin and soft tissue infections.
MethodParallel, randomized clinical trials randomly divided into “Control Group” (CG) and “Treatment Group” (TG), which were followed up for 10 consecutive days. Venous antibiotics and dressings were used in both groups. In addition, TOJT were used on the wounds in the TG. The outcome criteria were based on clinical indicators: Pressure Ulcer Scale for Healing (PUSH) and Visual Analog Scale Pain (VAS). The paired t-test or Wilcoxon, chi-squared or Fisher’s exact test, and Student’s t-test or Mann–Whitney tests were used with a significance level of 5%.
Results73 inpatients were included and followed up: 39 in TG and 34, CG. There were no significant differences in socio-demographic variables or of initial laboratory tests, except for blood glucose that was higher in TG than in CG (p = 0.044). Ten days into treatment, both the area of PUSH wounds (p < 0.001) and the pain scale (p = 0.029) were significantly reduced in TG. Staphylococcus aureus was the most prevalent pathogen (40%) with no significant difference between the two groups.
DiscussionAlthough the follow-up time was of only ten days, a significant improvement was observed in TG. As a limitation of the study, the small sample size precluded the comparison of S. aureus infections between the two groups.
ConclusionTOJT accelerated the healing process, reduced pain and contributed to an improvement in the clinical status of the wounds when compared to CG. These findings demonstrate the effectiveness and relevance of the employed technique. It can be easily incorporated as a routine procedure in hospitals without extra investment.
Infected surgical wounds are considered a public health problem worldwide with sizeable social and economic costs.1
In Brazil, the Ministry of Health has reported that in every minute two people undergo an amputation procedure due to small, infected lesions that had not been properly treated. This fact led to 17,000 amputations of legs and thighs in one year. However, these figures do not include the number of amputated toes and feet, which cost R$ 18.2 million (US $ 9.1 million) for the Public Health System in Brazil, or 38.4% of the total cost. Among amputees, about 70% of amputations were of diabetic feet.2
Infection is the invasion and multiplication of microorganisms in the tissue and inside the cells of a living being, as a result of causing continuity solution, leading to injuries, debilitation, and incapacitation.3
Wound infection triggers a cascade of events in the epidermis, dermis, and subcutaneous and adjacent tissues.4 The healing process consists of inflammatory, proliferative and remodeling phases with ultimate tissue restoration.1
Acute surgical wounds heal spontaneously within an expected period of time.5 In case of infection, the surgical wounds lose tissue, present with surgical dehiscences, i.e., the separation of the edges, which prevents the healing process. If no healing occurs within four weeks, the wound is considered chronic in which 60%–90% have a bacterial biofilm.6
At the site of the infected wound, there may be a sudden decrease in oxygen, which can go down to zero mmHg, thus hindering the repair process.4 In such environments, bacteria compete with the host cells for nutrients and may cause tissue destruction.4
Epidemiological data show that deep ulcers have a poor prognosis in terms of healing.7
Local infection can develop over 4–30 days, or even over period of one-year.3 About 40%–60% of non-traumatic lower limb amputations occur in diabetic patients, 85% of which are preceded by lesions on the feet.3,8 These rates are high due to complications caused by antibiotic-resistant microorganisms, which increases morbidity and mortality.9,10
The National Healthcare Safety Network (NHISN) estimates that, in the United States alone, 850,000 general surgeries were performed with a global incidence of surgical site infections (SSI) equal to 1.9%. In Brazil, the incidence of SSI in general and specific surgeries ranges from 1.45% to 38%.5 SSI leads to rising treatment costs for institutions, decreases bed turnover, prolongs length of hospital stay, and increases the risk of death.11
Among the procedures used for treating infected wounds, hyperbaric oxygen therapy (HBOT), developed in 1662 in England, should be highlighted.12,13
Oxygen concentration activates the immune cells, has a bactericidal function and it also aids collagen formation, angiogenesis, and cell mitosis in addition to repairing damaged tissues.7
In hyperbaric oxygen therapy (HBOT), the patient receives 100% inhaled oxygen in a closed setting environment, which activates the immune system and contributes to phagocytosis.7 However, the cost of this treatment is generally high, with US$163.00 being charged for each individual one-hour session in 2012 for a minimum period of 30 days. Each session corresponded to almost half the minimum wage at that time.14 The procedure was, therefore, very costly.15,16
In Brazil, the public health system – known in the country as SUS (Sistema Único de Saúde), has not provided patients with HBOT precisely due to its cost.9–13 It is available in private clinics with access restricted to those who can afford it.13 Diabetic patients, who frequently present with small wounds on their feet, have limited access to HBOT in Brazil.17
In 2015, topical oxygen therapy for the treatment of chronic wounds started being used again. Pressurized Topical Oxygen Wound therapy (TWO2) had been mentioned in a publication in 2003 by Gogia.18–20
The present study first conceived in 2005, when its main author discovered the technique during her daily routine working while providing care for a patient with perilesional dermatitis caused by a colostomy bag. Administering oxygen jets onto the hyperemic site quickly restored the injured area. This investigator has gained experience with technique by using it in her family members. In 2014 a clinical trial was started.
In Topical Oxygen Jet Therapy (TOJT), which is the focus of this study, oxygen is administered directly onto the wound. This intervention treats the infected wound, increases the oxygen level at the wound site, reducing infection and preparing it for possible surgeries and other types of treatment.
The pilot study was carried out in a hospital setting environment for one month; the cost of oxygen was calculated at US$2.49 per day/patient, i.e. US$75 per month, a much lower cost when compared to that of using HBOT which would cost US$ 4890 per month.
Thus, we aimed to evaluate the effectiveness of TOJT for treating infected surgical wounds and also to analyze the role of Staphylococcus aureus in these wounds.
MethodsThe study was designed as a parallel, randomized clinical trial. It was conducted at the Orthopedics and Traumatology Unit of a private, philanthropic health care institution in São Paulo, from April through December 2014.
The sample size was determined from a pilot study consisting of 10 cases, given that it is an unprecedented study for which there were no references in the literature. The outcome of interest was area of wound evolution assessed by the pressure ulcer scale; Student’s t-test was used to compare final and initial wound areas. Considering a significance level of 5% and a test power of 80%, the calculated sample size was 29 cases in each group. Additional patients were added to allow for possible losses of follow-up. A random list was prepared to allocate patients to one of the groups according to a first come, first served basis.
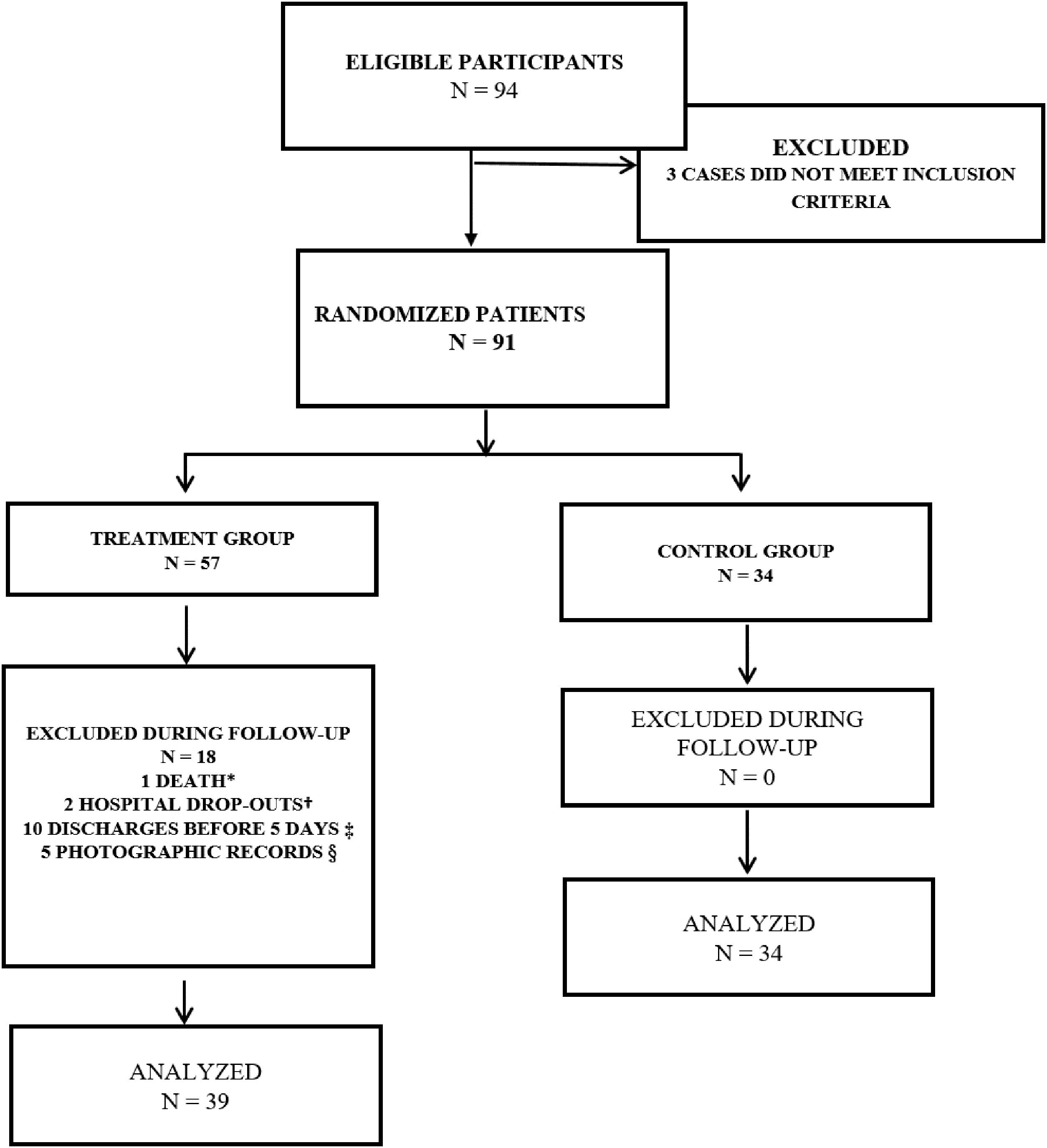
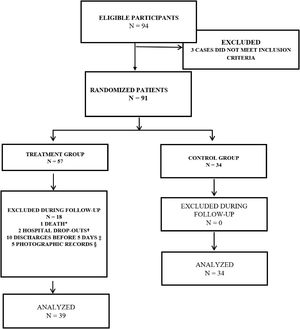
Patients were randomly allocated to Treatment Group and Control Group (Fig. 1). The study included adult patients having chronic wounds with clinical signs of surgical wounds infected for over 30 days, confirmed by microorganisms demonstration by means of biopsy, and presenting with phlogistic signs following surgical intervention. The groups were followed up for 10 consecutive days. Venous antibiotics and dressings were used in both groups. The Treatment Group also received TOJT on wounds.
Flowchart of the study participants. 2014.
* Death during surgical intervention; † Hospital drop-out due to fear of amputation; ‡ To assess the treatment effectiveness the participant had to remain hospitalized for at least 5 days; therefore, participants discharged before this period were excluded from the study; § Participants who were discharged without making the final photographic record for calculating their PUSH score.
Patients having drainage tube orifices, chronic wounds caused by arterial obstruction, and chronic obstructive pulmonary disease due to low oxygen concentration were excluded.
The study complied with national and international standards of ethics in research involving human beings, having obtained a favorable review from the local Research Ethics Committee (document Number: 2.288.875). All participants signed a voluntary informed consent form.
An Identification Form was used for collecting socio-demographic data and history of the disease and the wound. A Daily Form was used for following up patients’ general condition and the clinical status of their wounds; a Weekly Form for photographic records aimed at assessing wound size with the aid of the pressure ulcer scale; secretions from the wounds and blood samples for laboratory tests were collected.
Two instruments were used for assessing the treatment effectiveness: the Pressure Ulcer Scale for Healing (PUSH) and the Visual Analog Scale (VAS).
The validated PUSH is a clinical instrument developed by the National Pressure Ulcer Advisory Panel Task Force for assessing the wound evolution of healing.
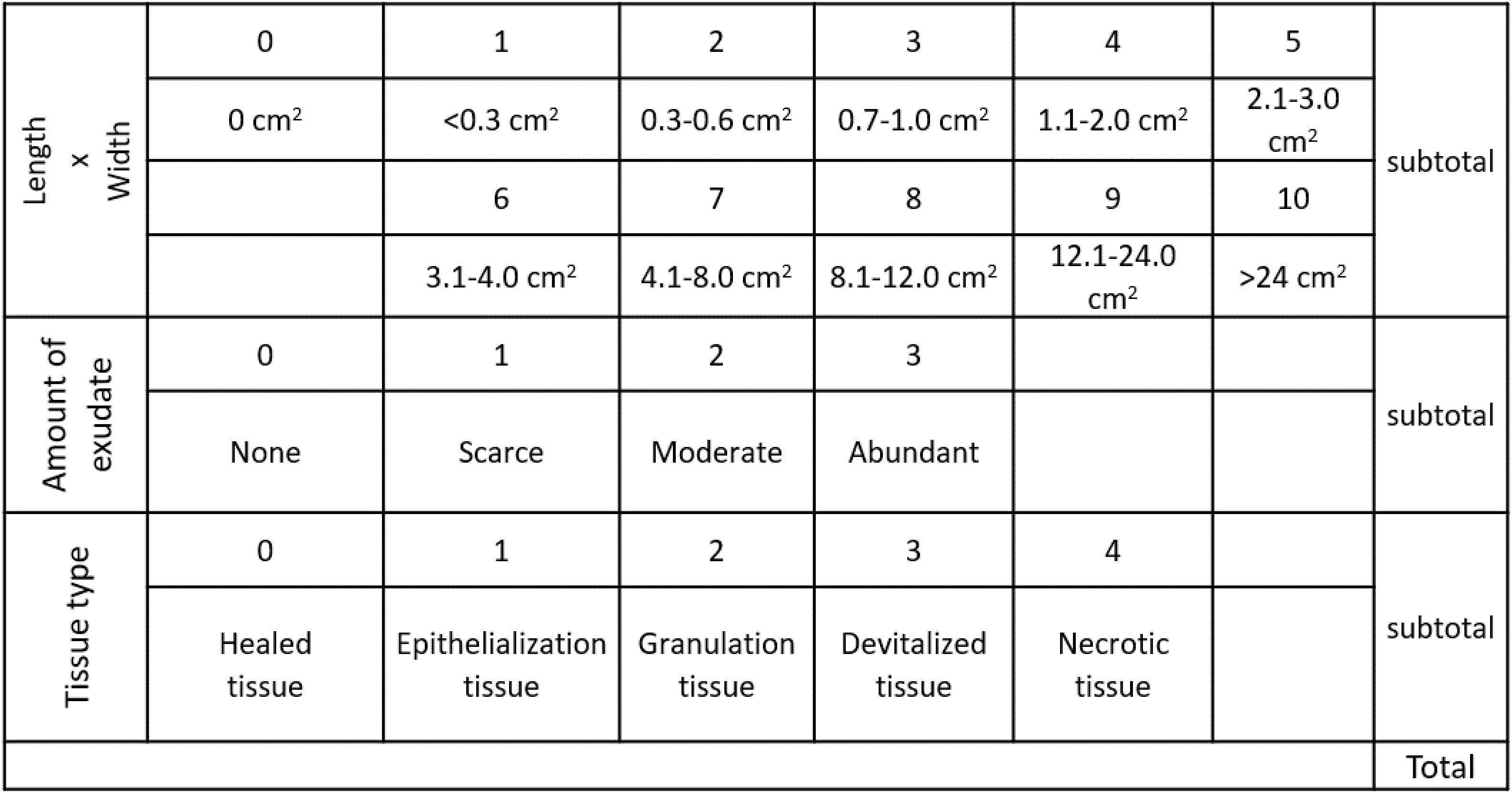
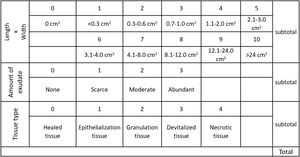
The wounds were photographed and the photographic records used for planimetry with a computerized device coupled to the application (UTHSCSA image-Too) to measure the irregular contours of the wounds from the initial moment until the end of the study 10 days thereafter.21 The wounds’ area (size and depth), secretions (amount and characteristics of the exudate) and type of tissue of the wounds’ bed are evaluated22,23 (Fig. 2).
The VAS24 assesses the intensity of pain reported by the patients.
Blood samples were collected at the beginning (day 1) and at the end (day 10) of the study. The following parameters were also measured: fasting blood glucose, serum albumin, leukogram, C-reactive protein, and erythrocyte sedimentation rate (ESR).25 All tests were performed by the Institution's own Central Laboratory.
Biological material swabs were collected from all patients from tissues suspected of being infected, preceded by cleaning with 0.9% saline jets and, in some cases, biopsy.26
During hospitalization, intravenous antibiotics were administered to patients according to the institution’s protocols and the results from quantitative and qualitative cultures in both groups with late surgical wounds.
TOJT was administered only to the Treatment Group. In the dressing, the wound aseptic procedure was identical for both groups and performed according to criteria that do not interfere with the normal healing process.
The dressing, changed once a day, consists of the following steps: (1) aseptic procedure of the lesion with warm 0.9% saline in abundance; the antiseptic used was a neutral glycerin soap for external use intended for showering and bathing, which was used for producing foam with 0.9% saline; this procedure was carried out from the least contaminated area to the most contaminated area and irrigation with 0.9% saline to remove dirt; (2) Essential Fatty Acids (EFA)26 applied onto the wound; (3) dressing and wound closure.
In the Treatment Group, in addition to the procedures mentioned above, the lesions were also treated with topical oxygen jets. The gas leaves the reservoir dry, at a 150 mmHg pressure and 15 L/min flow, travels inside the extension of a tubular latex catheter and hits the wound with an impact in every infected area at a minimum 0.5 cm distance and a maximum 5 cm distance, in order to ensure minimum safety distance and oxygen’s penetrating force. The administration time took 4–10 min, depending on the size of the wound. Therefore, it is neither risky nor invasive.
The Statistical Package for Social Sciences (SPSS) 13.0 (2004) was used in the statistical analysis. To assess the evolution of wounds in each group, Student’s t-test or Wilcoxon test were used for paired samples; categorical variables in each group were compared using Chi-squared test or Fisher’s Exact test, while for continuous variables Student’s t-test or Mann–Whitney test were used. A level of significance of 5% was adopted in the study.
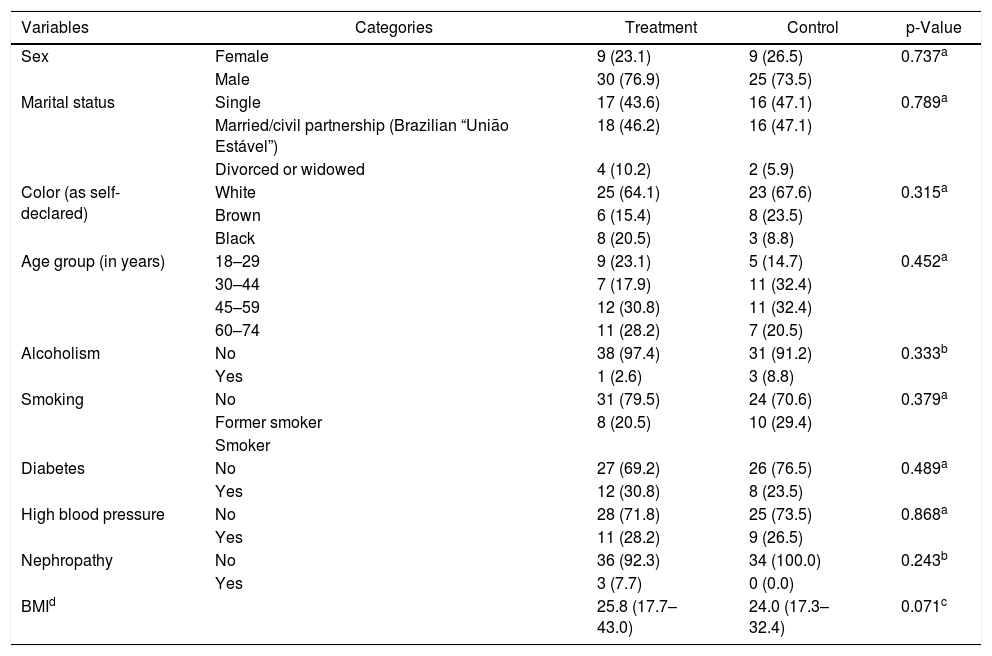
ResultsSeventy-three patients participated in the study, 39 (53.4%) in the Treatment Group and 34 (46.6%) in the Control Group, with both male and female participants, aged 18–74 years, with a mean of 47.3 (±16.6) years for the Treatment group and 45.4 (±14.3) years for the Control group. The socio-demographic and clinical characteristics of the investigated groups and the comparison between them are described in able 1. It is noteworthy that there was no significant difference between groups.
Also in Table 1, the investigated underlying conditions are shown. In the treatment group with 39 participants 12 were diabetic, 11 hypertensive, 3 nephropathic, and 13 had no associated pathologies. In the control group, of the 34 participants 8 were diabetics, 9 hypertensive, and 17 had no associated pathologies.
Socio-demographic variables of the 73 participants with infected surgical wounds according to study groups. São Paulo, SP, Brazil, 2014.
| Variables | Categories | Treatment | Control | p-Value |
|---|---|---|---|---|
| Sex | Female | 9 (23.1) | 9 (26.5) | 0.737a |
| Male | 30 (76.9) | 25 (73.5) | ||
| Marital status | Single | 17 (43.6) | 16 (47.1) | 0.789a |
| Married/civil partnership (Brazilian “União Estável”) | 18 (46.2) | 16 (47.1) | ||
| Divorced or widowed | 4 (10.2) | 2 (5.9) | ||
| Color (as self-declared) | White | 25 (64.1) | 23 (67.6) | 0.315a |
| Brown | 6 (15.4) | 8 (23.5) | ||
| Black | 8 (20.5) | 3 (8.8) | ||
| Age group (in years) | 18–29 | 9 (23.1) | 5 (14.7) | 0.452a |
| 30–44 | 7 (17.9) | 11 (32.4) | ||
| 45–59 | 12 (30.8) | 11 (32.4) | ||
| 60–74 | 11 (28.2) | 7 (20.5) | ||
| Alcoholism | No | 38 (97.4) | 31 (91.2) | 0.333b |
| Yes | 1 (2.6) | 3 (8.8) | ||
| Smoking | No | 31 (79.5) | 24 (70.6) | 0.379a |
| Former smoker | 8 (20.5) | 10 (29.4) | ||
| Smoker | ||||
| Diabetes | No | 27 (69.2) | 26 (76.5) | 0.489a |
| Yes | 12 (30.8) | 8 (23.5) | ||
| High blood pressure | No | 28 (71.8) | 25 (73.5) | 0.868a |
| Yes | 11 (28.2) | 9 (26.5) | ||
| Nephropathy | No | 36 (92.3) | 34 (100.0) | 0.243b |
| Yes | 3 (7.7) | 0 (0.0) | ||
| BMId | 25.8 (17.7–43.0) | 24.0 (17.3–32.4) | 0.071c |
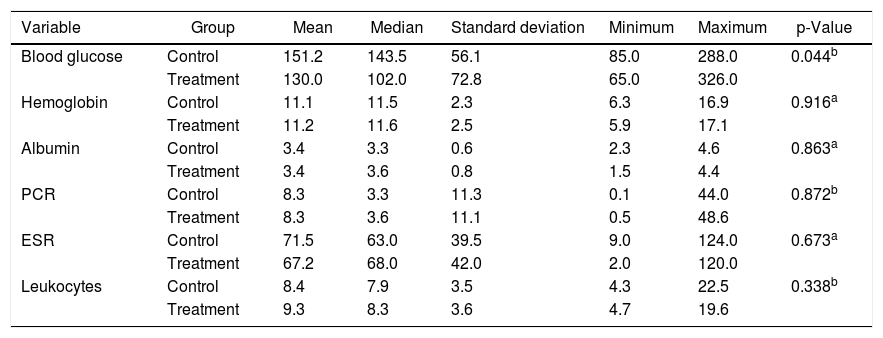
Regarding laboratory tests, the Treatment Group had higher mean blood sugar level than those found in the Control Group (p = 0.044). Other parameters were similar (Table 2).
Baseline laboratory tests of participants with surgical wounds infected according to study group, São Paulo, SP, Brazil, 2014.
| Variable | Group | Mean | Median | Standard deviation | Minimum | Maximum | p-Value |
|---|---|---|---|---|---|---|---|
| Blood glucose | Control | 151.2 | 143.5 | 56.1 | 85.0 | 288.0 | 0.044b |
| Treatment | 130.0 | 102.0 | 72.8 | 65.0 | 326.0 | ||
| Hemoglobin | Control | 11.1 | 11.5 | 2.3 | 6.3 | 16.9 | 0.916a |
| Treatment | 11.2 | 11.6 | 2.5 | 5.9 | 17.1 | ||
| Albumin | Control | 3.4 | 3.3 | 0.6 | 2.3 | 4.6 | 0.863a |
| Treatment | 3.4 | 3.6 | 0.8 | 1.5 | 4.4 | ||
| PCR | Control | 8.3 | 3.3 | 11.3 | 0.1 | 44.0 | 0.872b |
| Treatment | 8.3 | 3.6 | 11.1 | 0.5 | 48.6 | ||
| ESR | Control | 71.5 | 63.0 | 39.5 | 9.0 | 124.0 | 0.673a |
| Treatment | 67.2 | 68.0 | 42.0 | 2.0 | 120.0 | ||
| Leukocytes | Control | 8.4 | 7.9 | 3.5 | 4.3 | 22.5 | 0.338b |
| Treatment | 9.3 | 8.3 | 3.6 | 4.7 | 19.6 |
The types of surgical interventions performed were: surgical treatment of osteomyelitis 22 (30.1%) and ulcers in diabetic feet with amputations of toes, feet or lower limbs 20 (27.3%); surgical cleaning of external fixators 15 (20.5%) and orthopedic implants 9 (12.3%); bone tumors 3 (4.1%); injuries from firearms 2 (2.7%); injuries from bites in the upper limb 1 (1.35%); and sting injuries from venomous animal to the lower limb 1 (1.35%).
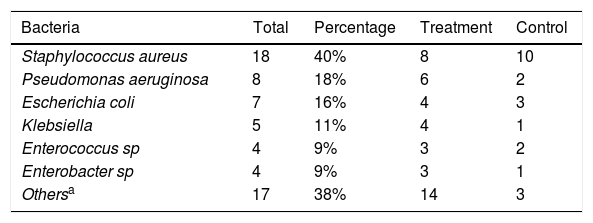
S. aureus was the most commonly identified bacterium (40%), with no significant difference between the two groups (Table 3).
Microbiological culture results of 45 infected secretions at baseline (day 1), São Paulo, SP, Brazil, 2014.
| Bacteria | Total | Percentage | Treatment | Control |
|---|---|---|---|---|
| Staphylococcus aureus | 18 | 40% | 8 | 10 |
| Pseudomonas aeruginosa | 8 | 18% | 6 | 2 |
| Escherichia coli | 7 | 16% | 4 | 3 |
| Klebsiella | 5 | 11% | 4 | 1 |
| Enterococcus sp | 4 | 9% | 3 | 2 |
| Enterobacter sp | 4 | 9% | 3 | 1 |
| Othersa | 17 | 38% | 14 | 3 |
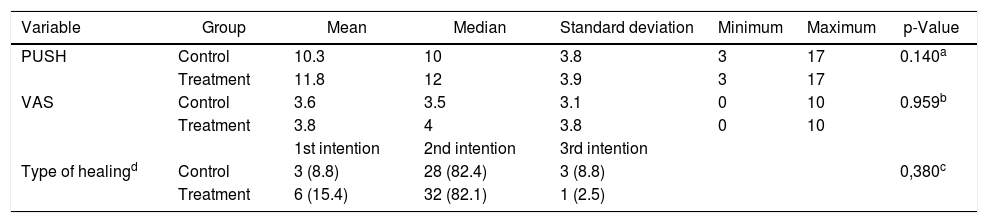
Wound severity was evaluated according to PUSH, VAS, and type of healing scales. The most common type of healing in both groups was that of secondary intention (about 82%), with no significant difference between groups (Table 4).
Variables related to the severity of wounds at baseline (day 1), São Paulo, SP, Brazil, 2014.
| Variable | Group | Mean | Median | Standard deviation | Minimum | Maximum | p-Value |
|---|---|---|---|---|---|---|---|
| PUSH | Control | 10.3 | 10 | 3.8 | 3 | 17 | 0.140a |
| Treatment | 11.8 | 12 | 3.9 | 3 | 17 | ||
| VAS | Control | 3.6 | 3.5 | 3.1 | 0 | 10 | 0.959b |
| Treatment | 3.8 | 4 | 3.8 | 0 | 10 | ||
| 1st intention | 2nd intention | 3rd intention | |||||
| Type of healingd | Control | 3 (8.8) | 28 (82.4) | 3 (8.8) | 0,380c | ||
| Treatment | 6 (15.4) | 32 (82.1) | 1 (2.5) |
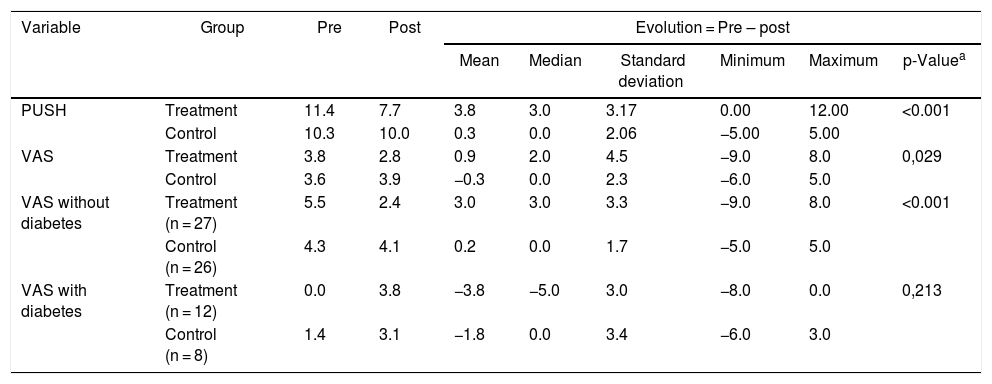
When comparing the initial and final wound condition in the Control Group there were neither significant changes in the PUSH scale nor in pain (VAS) scales over the 10-day period of observation. In contrast, the Treatment Group showed a statistically significant improvement in the PUSH scale (p < 0.001), while there was no difference in the VAS scale. Summary measures can be found in Table 5.
Pressure Ulcer Scale for Healing (PUSH) and the Visual Analog Scale (VAS) at baseline (day 1) and post-intervention (day 10) in the two groups. São Paulo, SP, Brazil, 2014.
| Variable | Group | Pre | Post | Evolution = Pre – post | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Standard deviation | Minimum | Maximum | p-Valuea | ||||
| PUSH | Treatment | 11.4 | 7.7 | 3.8 | 3.0 | 3.17 | 0.00 | 12.00 | <0.001 |
| Control | 10.3 | 10.0 | 0.3 | 0.0 | 2.06 | −5.00 | 5.00 | ||
| VAS | Treatment | 3.8 | 2.8 | 0.9 | 2.0 | 4.5 | −9.0 | 8.0 | 0,029 |
| Control | 3.6 | 3.9 | −0.3 | 0.0 | 2.3 | −6.0 | 5.0 | ||
| VAS without diabetes | Treatment (n = 27) | 5.5 | 2.4 | 3.0 | 3.0 | 3.3 | −9.0 | 8.0 | <0.001 |
| Control (n = 26) | 4.3 | 4.1 | 0.2 | 0.0 | 1.7 | −5.0 | 5.0 | ||
| VAS with diabetes | Treatment (n = 12) | 0.0 | 3.8 | −3.8 | −5.0 | 3.0 | −8.0 | 0.0 | 0,213 |
| Control (n = 8) | 1.4 | 3.1 | −1.8 | 0.0 | 3.4 | −6.0 | 3.0 | ||
When comparing the evolution between the Treatment Group and Control Group, a significant difference was observed with regard to PUSH (p < 0.001) and VAS (p = 0.029) scales in favor of Treatment Group (Table 5).
Once the presence of Diabetes Mellitus (DM) could have interfered with the sensation of pain (VAS) in the Treatment group, after adjusting for DM there was a reduction in pain in the Treatment Group and little change in the Control group (p < 0.001) [Table 5].
DiscussionThe discussion of the results is limited due to lack of published studies using oxygen jets applied directly onto late post-surgical infected wounds.
Topical oxygen jets delivered onto the wound is an active form of dressing;7,27 we observed during the study that oxygen played a fundamental role in tissue recovery.
The study groups had similar baseline socio-demographic and clinical profiles, except for blood glucose levels higher in patients of the Treatment Group. Another factor that can impact the healing process is the protein level, which was low in both groups, did not seem to have interfered with the performance of oxygen therapy.
In this study, the evolution of wounds (PUSH) from day 1 to day 10 in the Treatment Group was significantly better. The reduction of exudate, odor, pain, edema, heat, redness, and acceleration of the healing process were all observed in patients treated with oxygen. This demonstrates the contribution of TOJT to the healing process. On the other hand, no clinical improvement was observed in the Control Group.
The incidence of foot complications in individuals with DM in their lifetime is estimated between 15% and 25%. Three amputations every minute occur in people with DM worldwide.28 Patients with underlying diseases like DM have increased chances of developing complications and having delayed healing than those without an associated disease.8 In our study, all diabetic participants in the Treatment Group presented with a vitalized tissue.
Initially, all patients were analyzed without adjusting for DM. Nevertheless, although PUSH showed better results in the Treatment Group, VAS did not reflect the same finding, i.e. there was no difference between the two groups with regard to pain. Further adjusted analyses were carried out showing that in non-diabetic patients pain reduction was significantly greater in the Treatment Group compared to the Control Group. However, patients with DM as assessed by VAS had an increase in pain intensity from day1 to day 10 of the study in both groups, with no significant difference between them. We have interpreted such observations as an indication of improvement in sensitivity in the wounded area of diabetic feet as a result to treatments.
There is a particularity that was perceived in diabetic participants in the Treatment Group, namely an increase in pain and reduction in wound area over a shorter period of time, whereas the wounds of diabetic patients in the Control Group were pale, devitalized, necrotic, had an increase in size and were less sensitive, with few patients complaining of painful sensations.
Treatment with TOJT improved healing even in individuals with DM, as confirmed by PUSH, given that the sensation of pain may increase as a result of improved sensitivity.
The hyperoxygenation brought about by positive pressure on the infected wound diffuses into tissue and tunnels dug by bacteria, and adhere to neutrophils and macrophages, with unpaired oxygen fragments coupling to O2 and thereby originating superoxide (O2−).7,29 Superoxide, in turn, by gaining one more electron and two hydrogen ions (H), transforms into hydrogen peroxide (H2O2). Hydrogen peroxide receives extra electrons and is combined with hydrogen ions to form a hydroxyl radicals (−OH−). These radicals are lethal to most bacteria.7,29
Hyperoxygenation-activated neutrophils produce pathogen-destroying cytokines, decrease pain intensity, relax smooth muscles, assist endothelial cells, and are a vasodilator.7
Increase in oxygen tension in the body, especially at chronic wound sites, stimulates production of neutrophils and collagen, important mechanisms involved in local immunity and healing.7
Topical Oxygen Jet Therapy can be used against aerobic and anaerobic bacteria.30 Aerobic bacteria require host’s iron for growing, keeping it as Fe2+. Administered as topical jets, unpaired ions of oxygen binds to iron (Fe2+), thereby forming Fe3+ and going to an oxidized state, which is toxic to bacteria.30 In case of anaerobic bacteria, they tolerate up to 8% oxygen, but as concentration increases these bacteria die.30 The oxygen jet employed in this study is 100% oxygen, therefore lethal to anaerobic bacteria.
In our study, S. aureus was the most commonly identified bacterial species in the groups (40%). This finding is in agreement with previous reports, i.e. the microorganism mentioned above is the most frequent monobacterium in infected tissues, and is resistant to some antibiotics.3 The rates of wound infection caused by S. aureus in groups were not significantly different. Power to detect a relevant difference between groups was compromised by the small sample size, which is a limitation of the current study.
The mechanism of action of penicillins, cephalosporins, carbapenemics, monobactams and β-lactamase inhibitors consists of increasing osmotic pressure inside the bacterial cell, thus weakening and damaging the cell wall of Gram-positive and Gram-negative microorganisms.31
Resistance to β-lactamic agents has become an important clinical problem. Inadequate use of antibiotics and delay to initiate therapy contribute to microbial mutation and resistance.31
In our study, as oxygen molecules combine with hydrogen ions in the cell matrix and within the bacterial cell, they produce water (H2O), increase osmotic pressure inside the microorganism cell, and promote cell lysis.
Out of the chronic infected wounds that were difficult to heal, 53% presented with a biofilm, a substance produced by bacteria.6 During this trial, after treating infected wounds in patients with orthopedic prostheses, the presence of a large amount of viscous and hot clear liquid was observed, which lead us to think that the biofilm might have ruptured, but it needs further research.
Unlike the use of antibiotics, which requires timely administration, TOJT does not have this limitation. Even when the procedure was performed somewhat delayed, there were no observed changes in the clinical status of the wounds.
The temperature of the oxygen coming out from the reservoir is −183 °C. The region being treated keeps cold for some time; according to classic medical literature, pathogenic bacteria do not replicate at low temperatures, i.e. the procedure paralyzes the bacterial replication process.
The results from this study showed that use of Topical Oxygen Jet Therapy promotes bactericidal and bacteriostatic action. It also assists in the reconstruction of bone cells and muscle cells. In the necrotic area, more humidity was observed, favoring autolytic debridement, edema reduction, and local heat. It increased granulation tissue and formation of new blood vessels in the affected tissue. Odor elimination and pain reduction were also observed. Diabetics also slightly recovered their sensitivity.
Hyperbaric Oxygen Therapy (HBOT) is a well-known therapy used in the treatment of wounds. One of its main benefits is the proliferation of fibroblasts, neovascularization, osteoclastic and osteoblastic activity, and antimicrobial action,3 which also occur in TOJT. However, HBOT has a much higher cost than the TOJT as shown in this study. In addition, as TOJT is an individualized treatment, there is no risk of contamination and can be easily performed anywhere, from the ward itself to basic health units, or even at home.
TWO2 is another topical oxygen technique, also portable and associated with low costs when compared to HBOT. Still, its effectiveness is arguable.18,19 We understand that there are differences between TWO2 and TOJT.
No detailed description was found for TWO2; all we could ascertain is that it uses humidified oxygen in a continuous flow. On the other hand, TOJT works differently: dry gas comes out at a temperature of −183 °C, travels speedily at a positive pressure inside a catheter, falling impactfully onto the infected area, thereby penetrating tissue by means of a sweeping movement for some minutes, with the treated region being exposed to atmospheric air. TOJT uses existing resources of hospital settings, thus requiring no extra investment.
It should be pointed out that no adverse events were observed with the use of TOJT during the study. All patients received care from a single professional to ensure process standardization. It is worth mentioning that the saline solution for wound aseptic procedure was heated to 37 °C32,33 and used abundantly for better treatment results.
This clinical trial was carried out with inpatients; as this is a work in progress and without defined protocols at the moment, we were not able to control the length of hospital stay or to prevent medical co-interventions. Approximately 31% (18/57) of patients in the Treatment Group were unable to complete their treatment due to medical interventions; patients with afavorable evolution were discharged from hospital, therefore interrupting any new interventions. Conversely, in the Control Group, in turn, no patients were lost (Fig. 1).
Another difficulty faced when conducting the study was the shortage of antiseptic solutions to apply to the wounds; they needed to be replaced with neutral glycerin soap for external use intended for showering and bathing. However, no negative impact was noticed when this product was used on the participants’ wounds.
ConclusionThe use of Topical Oxygen Jet Therapy improved the clinical status of infected surgical wounds, and promoted improvements in the patients’ general health. Further studies could be conducted exploring the use of this technique in biofilm dissolution, treatment of other types of skin lesions, such as those caused by ionizing radiation, Hansen's bacillus (leprosy), ulcers due to arterial insufficiency, varicose ulcers, burns, and so on.
The Topical Oxygen Jet Therapy can optimize the healing process, decrease the length of hospital stay, increase bed turnover, and decrease hospital expenses. This therapy can be easily incorporated into routine procedures in hospitals without extra investment. Its implementation could possibly bring savings to the public health system.
FundingThis work was partially supported by CEALAG - CENTRO DE ESTUDOS AUGUSTO LEOPOLDO AYROSA GALVÃO.
Conflicts of interestThe authors declare no conflicts of interest.
It was conducted at the Orthopedics and Traumatology Unit of Irmandade da Santa Casa de Misericordia de Sao Paulo.