The aim of this study was to compare the trajectory of serogroups causing Invasive Meningococcal Disease (IMD) in the Santa Catarina (SC) state with those of whole Brazil. A retrospective analysis of all IMD cases reported from January 2007 to December 2019 was carried out. During the study period, 26,058 IMD cases were registered in Brazil and 644 and in SC state alone. Overall, Brazil showed progressive reduction in cases since 2010, when the meningococcal C conjugate vaccine was introducted on National Immunization Program, while SC showed an increase in total cases since 2013, particularly from serogroups W and C. Serogroups distribution was significantly different between Brazil and SC. The emergence of serogroup W highlights the improved meningococcal surveillance through increased accuracy in identification methods in SC state. This finding is important for discussing recommendations of quadrivalent (ACWY) conjugate vaccines in different geographical areas of Brazil.
Neisseria meningitidis (N. meningitidis) causes extremely severe conditions in humans, especially invasive meningococcal disease (IMD) characterized by one or more clinical syndromes including bacteremia, sepsis, or meningitis, the latter being the most common presentation.1
Capsular groups A, B, C, W, Y, and X cause almost all IMD.2 However, the quality and reliability of the information on IMD are not uniform across the world, due in part to differences in surveillance practices, use of different diagnostic methods and protocols, and application of different meningococcal case definition.3 Since late 1960s capsular group W has become a frequent cause of IMD,4 particularly in Europe, South America, Australia, and some parts of Sub-Saharan Africa.5
The highest incidence rates of IMD are observed in children aged less than one year,6,7 but in some regions other incidence peaks are observed among adolescents (16 through 21 years of age)8,9 and those aged ≥65 years.10,11 In Latin America, the incidence of IMD varies from <0.1 cases per 100,000 in countries including Mexico, Paraguay, Peru, and Bolivia to almost two cases per 100,000 in Brazil.7 In Brazil, N. meningitidis was the primary etiology of acute bacterial meningitis with serogroup C being the most prevalent in the country. In 2010, the National Immunization Program (NIP) included the MenC conjugate vaccine for children under two years old, with no catch-up campaign in older ages groups. Based on the significant IMD reduction observed only in the vaccinated group, the Ministry of Health decided to include MenC vaccine for adolescents aged 11–14 years in 2017.12
The introduction of multiplex real-time PCR (polymerase chain reaction) (RT-PCR) assay testing for S. pneumoniae, N. meningitidis and H. influenzae type B in sentinel hospitals in São Paulo increased the diagnostic yield for bacterial meningitis by 52, 85 and 20%, respectively, over culture-based methods.13 Since then, there has been a progressive increase in the use of PCR assays with improvement in microbiologic confirmation of bacterial meningitis throughout the country.
IMD is associated with substantial morbidity and overall case fatality rates (CFRs) of around 10% (with CFRs as high as 20% reported in some countries).14 Meningococcal disease caused by serogroup W alone has been associated with CFRs of >30%.15
Serogroups B and C are currently dominant in Latin America. Nevertheless, the proportion of IMD cases including outbreaks attributable to other serogroups have been reported in several countries.16 Southern Brazil, Argentina and Chile witnessed the emergence of MenW cc11 strain as a major cause of endemic meningococcal disease after 2003.17 The states of Parana, Santa Catarina and Rio Grande do Sul in the southern region of Brazil borders the southern cone countries of Latin America. In fact, W serogroup represented 17.8% of all invasive strains in 2003–2005 compared to 3.2% in 1995–2002.18
Notifications of IMD in Brazil are routinely collected by the Minister of Health trough the Information System for Notifiable Diseases (SINAN). This study is a retrospective review of all IMD cases reported in Brazil as a whole and in SC in particular, from 1 January 2007 to 31 December 2019, using the SINAN electronic database.19,20
The study variables included the number total cases of IMD and meningococcal serogroups. Pearson’s chi squared test was used to compare total cases and serogroups frequencies. For comparing distributions evolution, stacked bars were used as visualization. Statistical software used was R version 3.5.2.
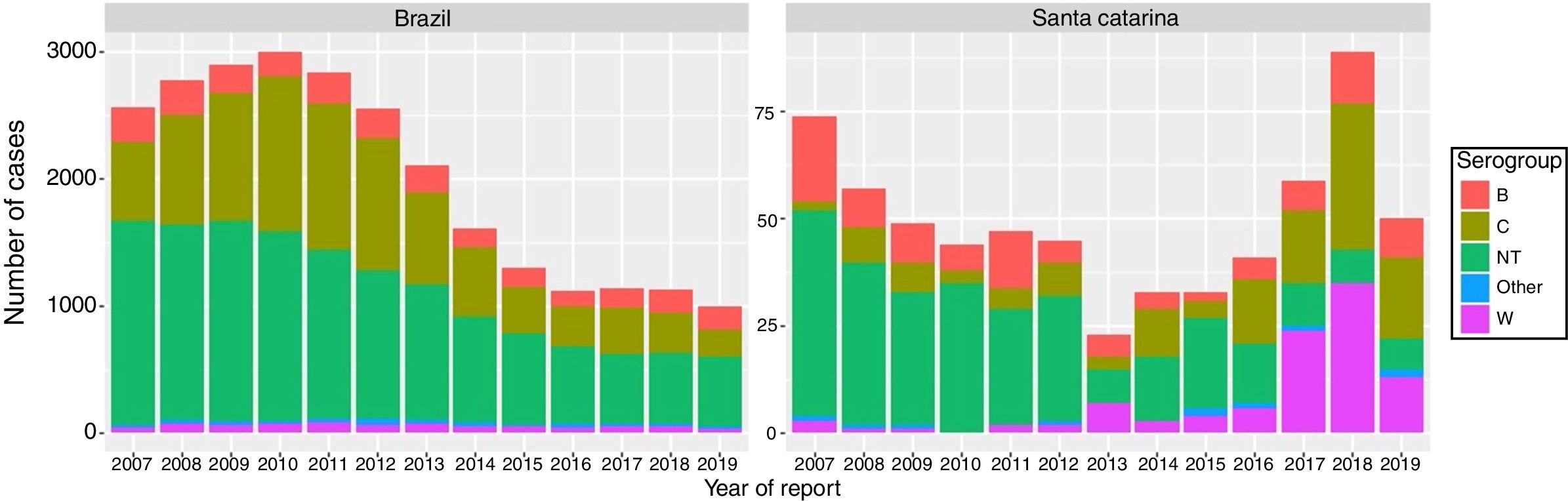
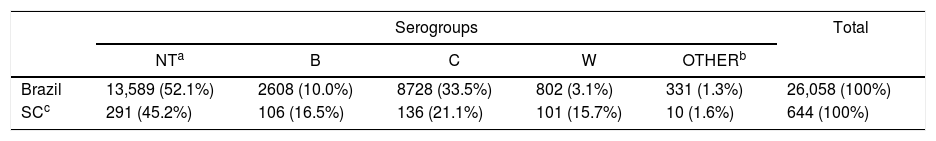
Total number of cases and cases according to serogroups were different between Brazil and Santa Catarina state along the reported years (Fig. 1). Analysis of total number of cases by year from 2007 through 2019 showed statistically significant differences between Brazil and SC state (p < 0.001) (Table 1).
A progressive decline of total cases and its respective serogroups occurred after introduction of meningococcal C vaccination in 2010 in Brazil. In contrast, there was a diminishing number of cases in SC state from 2007 to 2013, with a reversal of trends from 2014 to 2018, with a reduction of the number of the cases in 2019.
Serogroup distribution showed specific changes in SC state. Meningococcal disease by serogroup W increased between 2016–2018, with a reduction in 2019. Unlike Brazil, serogroup C showed an increment of cases after 2013 in SC state.
In general, non-identified cases showed decreasing trends in both regions even though they have occurred in different proportions.
Differences between the number of cases and distribution of serogroups cannot be clearly explained. There are several variables that can explain the changes in the prevalence of individual serogroups, one of them could be the naturally occurring periodic changes and another the impact of immunization programs. Another possibility, considering that SC is a region with high affluence of tourism (mainly from Argentina), is that these differences could be influenced by tourism carriage.
The notification of SC state presented a cut point at December 2nd, 2019, which might affect the analysis of the following months of the analyzed period.
The observed number of cases and serogroup distribution along time for SC state compared to Brazil, could imply that health authorities need to review current policies in order to cope with existing epidemiology, especially since the W serogroup is associated directly with increased mortality and morbidity rates.15,16
The emergence of serogroup W in SC highlights the difference in how health authorities have been dealing with meningococcal surveillance as the identification methods increased in accuracy. Moreover, it shows that geographical features should be considered as an important variable when recommending quadrivalent (ACWY) conjugate vaccines exceptionally considering the emergency of a strain that used to be underrepresented.
In summary, we showed that the total number of cases and serogroup distribution in the time period of 2007 through 2019 in SC state was significantly different compared to Brazil.
FundingSponsorship of this study and article processing charges were funded by Pfizer Inc.
Conflict of interestThe authors are consultant and speaker Pfizer, Sanofi, GlaxoSmithKline and Merck Sharp & Dohme.
Sponsorship of this study and article processing charges were funded by Pfizer Inc. All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published. The statistical analysis was conducted by Content Medicine in Buenos Aires, Argentina; funding was provided by Pfizer Inc. This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.