Renal replacement therapy is the treatment of end-stage chronic kidney disease and can be performed through dialysis catheters, arteriovenous fistulas/grafts, and peritoneal dialysis. Patients are usually immunocompromised and exposed to invasive procedures, leading to high rates of infection and increased mortality.
ObjectivesTo compare the prevalence of infection and related deaths, as well as the sensitivity profile of the putative bacteria in patients treated with peritoneal dialysis, arteriovenous fistula hemodialysis and catheter hemodialysis.
MethodsThis is case–control study. Six hundred forty-four patients undergoing renal replacement therapy were selected. Patients were divided into three groups according to the modality of dialysis treatment: peritoneal dialysis (126 patients), arteriovenous fistula hemodialysis (326 patients), and catheter hemodialysis (192 patients).
ResultsOne hundred sixteen patients (18.01%) developed infection. There was a higher incidence of infection in the peritoneal dialysis group (44 patients; 34.92%; OR: 3.32; CI 95%=2.13–5.17; p=0.0001). In the catheter hemodialysis group, 48 patients (25%) had infection (OR: 1.88; CI 95%: 1.24–2.85; p=0.0035). In the arteriovenous fistula hemodialysis group, 24 patients (7.36%) developed infection (OR: 0.19; CI 95%: 0.12–0.31; p=0.0001). Five patients (4.31%) died due to infection (four in the peritoneal dialysis group and one in the catheter hemodialysis group). There were no deaths due to infection in the arteriovenous fistula hemodialysis group.
ConclusionsPeritoneal dialysis is the treatment with greater risk of infection and mortality, followed by catheter hemodialysis. The lowest risk of infection and mortality was observed in arteriovenous fistula hemodialysis group.
Chronic kidney disease (CKD) has high prevalence and incidence worldwide, particularly in Brazil.1 Although the National Kidney Foundation (NKF)2 in 2002 has proposed staging CKD in order to slow the advance toward the functional failure of the kidneys, difficulties such as lack of early diagnosis, inadequate treatment in the early stages, delayed specialized monitoring, and the complexity of the disease lead many people to need renal replacement therapy (RRT).1–3 RRT can be implemented through hemodialysis (HD), peritoneal dialysis (PD), and renal transplantation. Each treatment has its own characteristics, advantages, disadvantages, and complications.4 Peritoneal dialysis allows the patient home treatment decreasing outpatient visits. Hemodialysis can be performed through a central catheter (CH) inserted in the internal jugular or subclavian vein or through an arteriovenous fistula (AVF) preferably in the upper limbs whose optimal functionality delay varies from one to three months.5,6 Nosocomial infection is one of the most serious complications and the second cause of death in dialysis patients.7 The risk factors that predispose to nosocomial infection in RRT may be influenced by patient characteristics, site of dialysis access, and disorders of the skin and mucous membranes8; and comorbidities such as diabetes mellitus, anemia, cardiovascular disease, immunosuppression, and metabolic imbalances.4,5 Peritonitis is the most frequent infection in patients undergoing PD, and septicemia is the most frequent complication among patients on HD, especially when conducted through a central venous catheter. The kind of vascular access for HD has significant influence on patient survival. Catheters are associated with substantially greater risk of septicemia, hospitalization, and mortality compared to AVF.9 There are few studies in Brazil evaluating infection rates, the prevalent microorganisms and the susceptibility profile of bacterial infections associated with RRT. These data may be useful for empirical anti-infective therapy in these patients, as well as to better evaluate the choice of dialysis treatment aiming at preventing infections. The objective of the present study was to compare the prevalence of infection and related deaths, as well as the sensitivity profile of the putative bacteria in RRT treated patients.
MethodsThis is a case–control study. The study sample consisted of 644 patients treated in the RRT outpatient center at Santa Casa de Misericordia de Ponta Grossa Hospital during a 29-month period. Patients on RRT who developed nosocomial infection (116 patients) were considered as cases. Patients who did not develop infection (528 patients) were considered as controls. Nosocomial infections were considered when the Commission of Hospital Infection Control (CHIC) identified the case as such, based on clinical features, complementary blood tests, and culture results of biological material. Unconfirmed cases of infection by the CHIC were excluded. The patients were divided into three groups according to the type of dialysis treatment: PD (116 patients), CH (192 patients), and arteriovenous fistula hemodialysis (AVH) (326 patients). The study was approved by the local ethics committee.
Statistical analysisTo compare categorical variables, two-tailed Fisher's exact test with Bonferroni correction for multiple comparisons was used. To evaluate the effect size Odds Ratio (OR) was calculated with 95% confidence interval (CI) and its transformation in probability (p). The samples were properly tested for normality by Anderson–Darling test. The presence of outliers was checked by the Grubbs test. The statistical power of the sample was computed in each comparison. Results are presented as mean±standard deviation (SD). Analyses, including descriptive statistics, were performed using EPI INFO program. p<0.05 was considered statistically significant.
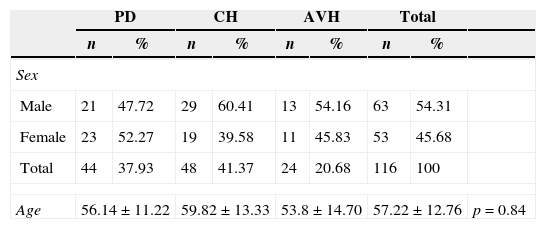
ResultsOf the 644 patients who underwent dialysis during the study period, 116 (18.1%) developed some infection (63 male; 57.22±12.76 years). One hundred fifty-six infections were reported. Seventy-one (45.51%) infections (56.14±11.22 years) were in patients on PD, 60 (38.46%) infections (59.82±13.33 years) in patients on CH, and 25 (16.2%) infections in patients on AVH (53.8±14.7 years) (Table 1).
Patient characteristics by age and sex.
| PD | CH | AVH | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| Sex | |||||||||
| Male | 21 | 47.72 | 29 | 60.41 | 13 | 54.16 | 63 | 54.31 | |
| Female | 23 | 52.27 | 19 | 39.58 | 11 | 45.83 | 53 | 45.68 | |
| Total | 44 | 37.93 | 48 | 41.37 | 24 | 20.68 | 116 | 100 | |
| Age | 56.14±11.22 | 59.82±13.33 | 53.8±14.70 | 57.22±12.76 | p=0.84 | ||||
PD, peritoneal dialysis; CH, central catheter hemodialysis; AVH, arteriovenous fistula hemodialysis.
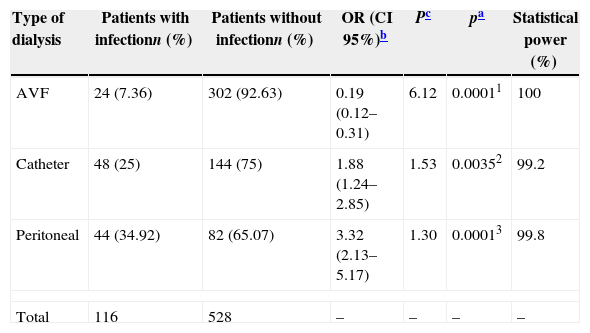
Of the 126 patients undergoing PD, 44 (34.92%) had at least one infection during the study period, which was significantly higher than that in the other two groups (OR: 3.32; CI 95%: 2.13–5.17; p=0.0001). CH also showed up as a risk factor for the development of infection with 48 (25%) of the 192 infected patients (OR: 1.8824; CI 95%: 1.24–2.85; p=0.0035). The lowest incidence of infection was observed in AVH group: 24 (7.36%) of 326 patients (OR: 0.19; CI 95%: 0.12–0.35; p=0.0001). For all comparisons made, the statistical power was greater than 99%, with a consequent error – beta less than 1% (Table 2).
Rate of infection in patients on renal replacement therapy according to the type of dialysis.
| Type of dialysis | Patients with infectionn (%) | Patients without infectionn (%) | OR (CI 95%)b | Pc | pa | Statistical power (%) |
|---|---|---|---|---|---|---|
| AVF | 24 (7.36) | 302 (92.63) | 0.19 (0.12–0.31) | 6.12 | 0.00011 | 100 |
| Catheter | 48 (25) | 144 (75) | 1.88 (1.24–2.85) | 1.53 | 0.00352 | 99.2 |
| Peritoneal | 44 (34.92) | 82 (65.07) | 3.32 (2.13–5.17) | 1.30 | 0.00013 | 99.8 |
| Total | 116 | 528 | – | – | – | – |
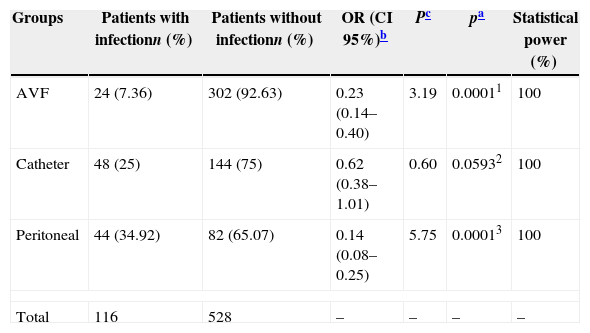
Comparing the groups individually, AVH turned out to be the safest method with less infections, resulting in lower incidence of morbidity when compared with CH (OR: 0.23; CI 95%: 0.14–0.40; p=0.0001) and PD (OR: 0.14; CI 95%: 0.08–0.25; p=0.0001). There was no statistically significant difference between the incidence of infections in the PD group and the CH group (OR: 0.62; CI 95%: 0.38–1.01; p=0.0593). The statistical power to detect these differences was 100% in both cases, therefore no beta error (Table 3).
Comparison of the infection frequency in the different groups of dialysis.
| Groups | Patients with infectionn (%) | Patients without infectionn (%) | OR (CI 95%)b | Pc | pa | Statistical power (%) |
|---|---|---|---|---|---|---|
| AVF | 24 (7.36) | 302 (92.63) | 0.23 (0.14–0.40) | 3.19 | 0.00011 | 100 |
| Catheter | 48 (25) | 144 (75) | 0.62 (0.38–1.01) | 0.60 | 0.05932 | 100 |
| Peritoneal | 44 (34.92) | 82 (65.07) | 0.14 (0.08–0.25) | 5.75 | 0.00013 | 100 |
| Total | 116 | 528 | – | – | – | – |
Of patients infected (116), five died due to infection, with an overall mortality rate of 4.31%. There were four deaths (9.09%) in PD group and one (8.2%) in CH group. No death was observed in AVH group.
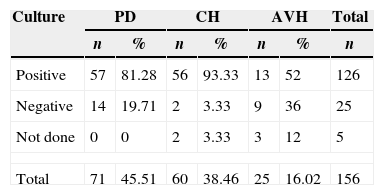
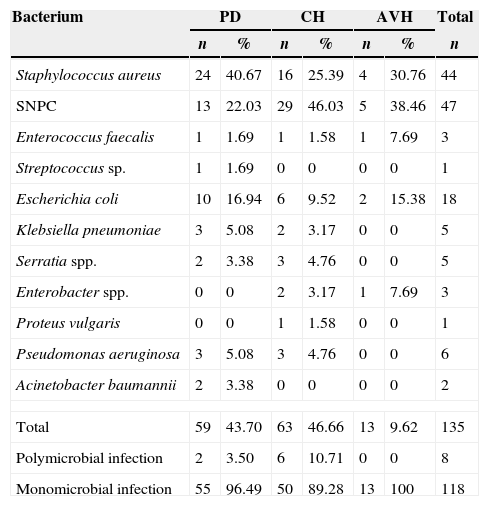
Sites of infection and bacterial etiologyMicrobiological tests were carried out in 151 (96.78%) patients, with infections turning out positive in 126 (80.76%). CH group was the group with the highest positivity rate (93.33%; 56/58 cultures), followed by the PD group (81.28%; 59/71 cultures) and the AVH group (52%; 13/22 cultures). The biological samples used for culture were peritoneal fluid in 46.47% of infections of PD group, blood culture in 72.41% of infections of CH group and 40.9% of infections of AVH group. Other samples used for culture were catheter secretion, dialysate fluid, cardiac valve secretion, sputum, and urine (Table 4). The most common infection in the PD group was peritonitis in 55 (77.46%) patients. In CH group, bloodstream infection was the most frequent infection occurring in 44 (73.33%) patients. Vascular access was the commonest site of infection in AVH group occurring in 12 (48%) patients (Table 5). Staphylococcus aureus was the most commonly recovered bacteria present in 44 (32.59%) cases (24 in PD group, 16 in CH group, and 4 in AVH group). Coagulase-negative Staphylococcus spp. (SNPC) was recovered from 47 (34.81%) cases (13 in PD group, 29 in CH group, and 5 in AVH group). Escherichia coli was isolated in 18 (13.33%) cases (10 in PD group, 6 in CH group, and 2 in AVH group). Other less frequent bacteria are shown in Table 6.
Microbiological culture results from patients on different types of dialysis.
| Culture | PD | CH | AVH | Total | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | |
| Positive | 57 | 81.28 | 56 | 93.33 | 13 | 52 | 126 |
| Negative | 14 | 19.71 | 2 | 3.33 | 9 | 36 | 25 |
| Not done | 0 | 0 | 2 | 3.33 | 3 | 12 | 5 |
| Total | 71 | 45.51 | 60 | 38.46 | 25 | 16.02 | 156 |
PD, peritoneal dialysis; CH, central catheter hemodialysis; AVH, arteriovenous fistula hemodialysis.
Site of infection in patients on different types of dialysis.
| PD | CH | AVH | Total | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Site | ||||||||
| Peritonitis | 55 | 77.46 | 0 | 0 | 0 | 0 | 55 | 35.25 |
| Blood stream (BS) | 3 | 4.22 | 44 | 73.33 | 4 | 16 | 51 | 32.69 |
| Related to vascular access/catheter | 12 | 16.90 | 14 | 23.33 | 12 | 48 | 38 | 24.35 |
| Urinary tract infection (UTI) | 0 | 0 | 2 | 3.33 | 2 | 8 | 4 | 2.5 |
| Respiratory tract infection | 0 | 0 | 0 | 0 | 4 | 16 | 4 | 2.5 |
| Endocarditis | 0 | 0 | 0 | 0 | 3 | 12 | 3 | 1.92 |
| Peritonitis+BS+UTI | 1 | 1.40 | 0 | 0 | 0 | 0 | 1 | 0.64 |
| Total | 71 | 45.51 | 60 | 38.46 | 25 | 16.02 | 156 | 100 |
PD, peritoneal dialysis; CH, central catheter hemodialysis; AVH, arteriovenous fistula hemodialysis.
Etiology of infections from patients on different types of dialysis.
| Bacterium | PD | CH | AVH | Total | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | |
| Staphylococcus aureus | 24 | 40.67 | 16 | 25.39 | 4 | 30.76 | 44 |
| SNPC | 13 | 22.03 | 29 | 46.03 | 5 | 38.46 | 47 |
| Enterococcus faecalis | 1 | 1.69 | 1 | 1.58 | 1 | 7.69 | 3 |
| Streptococcus sp. | 1 | 1.69 | 0 | 0 | 0 | 0 | 1 |
| Escherichia coli | 10 | 16.94 | 6 | 9.52 | 2 | 15.38 | 18 |
| Klebsiella pneumoniae | 3 | 5.08 | 2 | 3.17 | 0 | 0 | 5 |
| Serratia spp. | 2 | 3.38 | 3 | 4.76 | 0 | 0 | 5 |
| Enterobacter spp. | 0 | 0 | 2 | 3.17 | 1 | 7.69 | 3 |
| Proteus vulgaris | 0 | 0 | 1 | 1.58 | 0 | 0 | 1 |
| Pseudomonas aeruginosa | 3 | 5.08 | 3 | 4.76 | 0 | 0 | 6 |
| Acinetobacter baumannii | 2 | 3.38 | 0 | 0 | 0 | 0 | 2 |
| Total | 59 | 43.70 | 63 | 46.66 | 13 | 9.62 | 135 |
| Polymicrobial infection | 2 | 3.50 | 6 | 10.71 | 0 | 0 | 8 |
| Monomicrobial infection | 55 | 96.49 | 50 | 89.28 | 13 | 100 | 118 |
PD, peritoneal dialysis; CH, central catheter hemodialysis; AVH, arteriovenous fistula hemodialysis.
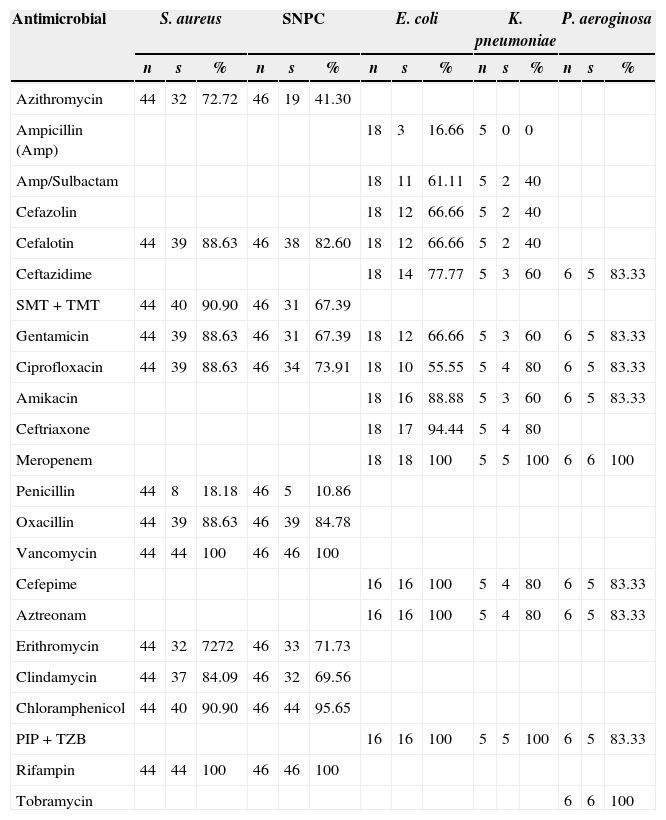
The sensitivity profiles of the main isolated bacteria are shown in Table 7. Among the most important findings, 5 (11.4%) methicillin-resistant S. aureus (MRSA) isolates, all observed in PD group, and 7 (15.2%) methicillin-resistant SNPC infections (6 in CH group) stand out. There was no reported resistance to neither vancomycin nor rifampicin. One (5.5%) E. coli isolate from PD group was extended-spectrum β-lactamase producer (ESBL) and multidrug resistant to antimicrobials. Multidrug resistant Klebsiella pneumoniae was recovered from two isolates (40%), one from PD group and another from CH group. Isolates of Serratia spp. (5) were susceptible to most antimicrobials tested. Resistant Pseudomonas aeruginosa was observed in only one isolate (16.7%) from CH group. Carbapenem resistance was not detected. The remaining isolates were susceptible to most antibiotics tested. Overall, multidrug resistant bacterial infections were recovered from 16 (10.26%) patients: 9 (56.25%) from CH group and 7 (43.75%) from PD group, representing 14.28% and 11.86% infections, respectively, with no statistical difference (OR: 1.23; CI 95%: 0.42–3.5; p=0.7914; statistical power of 100%). No multidrug resistant isolate was recovered from AVH group.
Sensitivity profile of the main isolated bacteria.
| Antimicrobial | S. aureus | SNPC | E. coli | K. pneumoniae | P. aeroginosa | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | s | % | n | s | % | n | s | % | n | s | % | n | s | % | |
| Azithromycin | 44 | 32 | 72.72 | 46 | 19 | 41.30 | |||||||||
| Ampicillin (Amp) | 18 | 3 | 16.66 | 5 | 0 | 0 | |||||||||
| Amp/Sulbactam | 18 | 11 | 61.11 | 5 | 2 | 40 | |||||||||
| Cefazolin | 18 | 12 | 66.66 | 5 | 2 | 40 | |||||||||
| Cefalotin | 44 | 39 | 88.63 | 46 | 38 | 82.60 | 18 | 12 | 66.66 | 5 | 2 | 40 | |||
| Ceftazidime | 18 | 14 | 77.77 | 5 | 3 | 60 | 6 | 5 | 83.33 | ||||||
| SMT+TMT | 44 | 40 | 90.90 | 46 | 31 | 67.39 | |||||||||
| Gentamicin | 44 | 39 | 88.63 | 46 | 31 | 67.39 | 18 | 12 | 66.66 | 5 | 3 | 60 | 6 | 5 | 83.33 |
| Ciprofloxacin | 44 | 39 | 88.63 | 46 | 34 | 73.91 | 18 | 10 | 55.55 | 5 | 4 | 80 | 6 | 5 | 83.33 |
| Amikacin | 18 | 16 | 88.88 | 5 | 3 | 60 | 6 | 5 | 83.33 | ||||||
| Ceftriaxone | 18 | 17 | 94.44 | 5 | 4 | 80 | |||||||||
| Meropenem | 18 | 18 | 100 | 5 | 5 | 100 | 6 | 6 | 100 | ||||||
| Penicillin | 44 | 8 | 18.18 | 46 | 5 | 10.86 | |||||||||
| Oxacillin | 44 | 39 | 88.63 | 46 | 39 | 84.78 | |||||||||
| Vancomycin | 44 | 44 | 100 | 46 | 46 | 100 | |||||||||
| Cefepime | 16 | 16 | 100 | 5 | 4 | 80 | 6 | 5 | 83.33 | ||||||
| Aztreonam | 16 | 16 | 100 | 5 | 4 | 80 | 6 | 5 | 83.33 | ||||||
| Erithromycin | 44 | 32 | 7272 | 46 | 33 | 71.73 | |||||||||
| Clindamycin | 44 | 37 | 84.09 | 46 | 32 | 69.56 | |||||||||
| Chloramphenicol | 44 | 40 | 90.90 | 46 | 44 | 95.65 | |||||||||
| PIP+TZB | 16 | 16 | 100 | 5 | 5 | 100 | 6 | 5 | 83.33 | ||||||
| Rifampin | 44 | 44 | 100 | 46 | 46 | 100 | |||||||||
| Tobramycin | 6 | 6 | 100 | ||||||||||||
As previously reported, PD patients had higher rate of infection compared to CH and AVH patients.9–12 Likewise, a high mortality rate (9.9%) of infected patients was observed in PD group when compared to CH patients (2.08%). Johnson et al.13 reported a mortality rate of 7.6% and 4.2%, respectively, also showing an increased risk of death in infected PD patients. Lower rates (3.5%) of mortality in PD were reported by Barretti et al.14 Peritonitis is the main limitation of PD and is very frequent.11,13–15 In CH group, bloodstream infection is the most common site of infection. In the present study, bloodstream infection was presented in 73.33% of patients in CH group, compared to 16% in AVH group and 4.22% in PD group. These results are similar to several studies reporting 50–80% of bloodstream infections16 mostly related to the use of catheters.11,12,16 Couto et al.15 describe aseptic peritonitis, an entity found in about 10% of cases, where there is no identified etiological agent. In the present study 19.71% of negative cultures were found in PD group with clinically diagnosed peritonitis. Furthermore, culture was made in all cases with no possibility of underreporting. The most frequent infectious agents isolated were Gram positive bacteria responsible for 70.36% of all infections. Similar values are found in the study by Silva et al.6 with 60% of cases, in Aslan et al.11 with 62%, and in Couto et al.15 with 67% of cases. Other studies show that the proportion of Gram positive and Gram negative bacteria is similar.17,18S. aureus and SNPC were the most frequent pathogens isolated, as in other studies.14,15,19–21 Methicillin resistant SNPC was found in 15.2% of infections, a lower rate of resistance than the 58.4% and 100% reported by Barretti et al.14 and Britta et al.,20 respectively. The incidence of MRSA in the present study was 11.4% compared to rates of 14.3%,14 22%,22 23%,20 and 43.4%17 found in other studies. Gram negative organisms were responsible for 23.69% of all infections in the present study. E. coli was the most frequent with 13.33%. Seventeen of 18 cultures (94.45%) were sensitive to ceftriaxone, whereas 66.7% and 55.6% were sensitive to ciprofloxacin and cefalotin. Britta et al.20 reported a study in which E. coli had 100% resistance to these antibiotics. An isolate of E. coli was ESBL producer (5.55%), multidrug resistant, and sensitive to meropenem. As in the study by Sader et al.23 no resistance to this antibiotic was found in the present study. For K. pneumoniae 80% was sensitive to ceftriaxone and 60% to ceftazidime. Nogueira et al.24 showed 53.3% and 80% sensitivity, respectively, and Almeida et al.25 reported 34% sensitivity for both antibiotics. Serratia, Enterobacter, and Proteus were also isolated (Table 6). Non-fermentative bacilli were isolated in eight (5.92%) cultures, P. aeruginosa in six (4.44%) of which only one (16.7%) was multidrug resistant, and Acinetobacter baumannii was recovered in two cultures. Britta et al.20 showed multidrug resistance in 50% of cases. Polymicrobial infections were observed in 3.5% of cases of PD group, a rate much lower than the 30%,15 10.7%,18 and 9.09%25 reported by other studies. In CH group polymicrobial infections were more frequently isolated (10.71%). The lower rate of resistance of the isolates in the present study translates into reduced morbidity and hospital costs.26 This finding could be attributed to the antimicrobial control policy implemented by the CHIC, which is based on quarterly updates of clinical sensitivity of local microbiota and prescription supervision of electronic medical records.
ConclusionsThe present study provides evidence that the type of vascular access in RRT is an important predisposing factor for infection which, in turn, increases comorbidity and hospital costs. The AVF for hemodialysis is the safest access for RRT. These results emphasize the need for early referral program for patients with CKD regarding the indication and maturation of the dialysis method. This policy decreases morbidity, mortality, and hospital costs. More studies are needed, especially those that are prospective and adequately powered.
Conflicts of interestThe authors declare no conflicts of interest.
The authors thank the clinical staff of the center of renal replacement therapy of the Santa Casa de Misericórdia de Ponta Grossa hospital who kindly agreed to collaborate with data access: nurse Karla Andréia Santos, Merluci Days, and Ms Adriana Aparecida Fabri Beneduzzi; the hospital teaching sector: Almir José Martins, Adriana Fátima Menegat Schunski and Glaucia Aparecida Alves; and the hospital infection control service: Thais Caroline Rodrigues de Oliveira.