Vibrio vulnificus causes an infectious disease that has extremely poor convalescence and leads to necrotic fasciitis. In this study, we sought to define the characteristic epidemiology of V. vulnificus infection and clarify its diagnosis at the global level.
MethodsOver a period of 10 years, we investigated the appearance of symptoms, underlying conditions, treatment, and mortality in 12 patients (eight men, four women; >50 years old; average age, 66 years,) infected with V. vulnificus.
ResultsThe development of symptoms occurred primarily between June and September, a period during which seawater temperature rises and the prevalence of V. vulnificus increases. All patients had underlying diseases, and seven patients reported a history of consuming fresh fish and uncooked shellfish. The patients developed sepsis and fever with sharp pain in the limbs. Limb abnormalities were observed on visual examination. All patients underwent debridement; however, in the survival group, the involved limb was amputated early in 80% patients. The mortality rate was 58.3%.
ConclusionRecognition of the characteristic epidemiology and clinical features of this disease is important, and positive debridement should be performed on suspicion. When the illness reaches an advanced stage, however, amputation should be the immediate treatment of choice.
Vibrio vulnificus is a Gram-negative bacillus identified by Farmer in 1979. It is present in coastal seawater, and induces symptoms of necrotizing fasciitis.1 Until recently, V. vulnificus infection was restricted to brackish water regions with warm weather and low sea salt concentration. For example, 40% of all V. vulnificus-infected individuals live in the northern region of Kyushu, which is adjacent to the Ariake Sea, a shallow brackish water region. However, global warming in recent years has increased sea temperature, and the symptoms of V. vulnificus infection have been observed in areas not endemic to this infection.2 Despite its high fatality rate, V. vulnificus infection is rare, and knowledge about this infection is low. By re-examining the accumulated cases at our hospital, we wish to investigate the diagnosis and treatment of V. vulnificus infection and provide information regarding this at the global level.
Research purposeBy investigating recently infected patients at our hospital, we sought to clarify the clinical features and route of V. vulnificus infection. Patients were divided into a death group and a survival group to investigate the differences in treatment. A flow chart from diagnosis to treatment of V. vulnificus infection was then prepared.
Patients and methodsTwelve patients with V. vulnificus-induced necrotic fasciitis, who consulted our department between January 2001 and December 2010, were included in this retrospective study based on patients’ clinical records. Patient characteristics and route of infection were investigated, followed by establishing a relationship between the month of symptom onset and seawater temperature in that month; a graph was created based on the results (Study 1). Physical and laboratory findings at the first medical examination were analyzed (Study 2). The patients were divided into a survival group and a death group to investigate differences in treatment (Study 3). Medical treatments primarily included antibiotic administration, debridement, and limb amputation. Differences in the time between calling for an ambulance and treatment initiation were examined. History of raw seafood consumption, which was reported by the family, was ambiguous, and the time from consuming uncooked seafood to the development of symptoms differed among patients; some patients may have had an incubation period of several days. Considering that the patients would call an ambulance on appearance of severe symptoms, which also indicates increased bacilli in the blood, the time of calling for an ambulance was considered as disease onset. Subsequently, the time between disease onset and death was investigated.
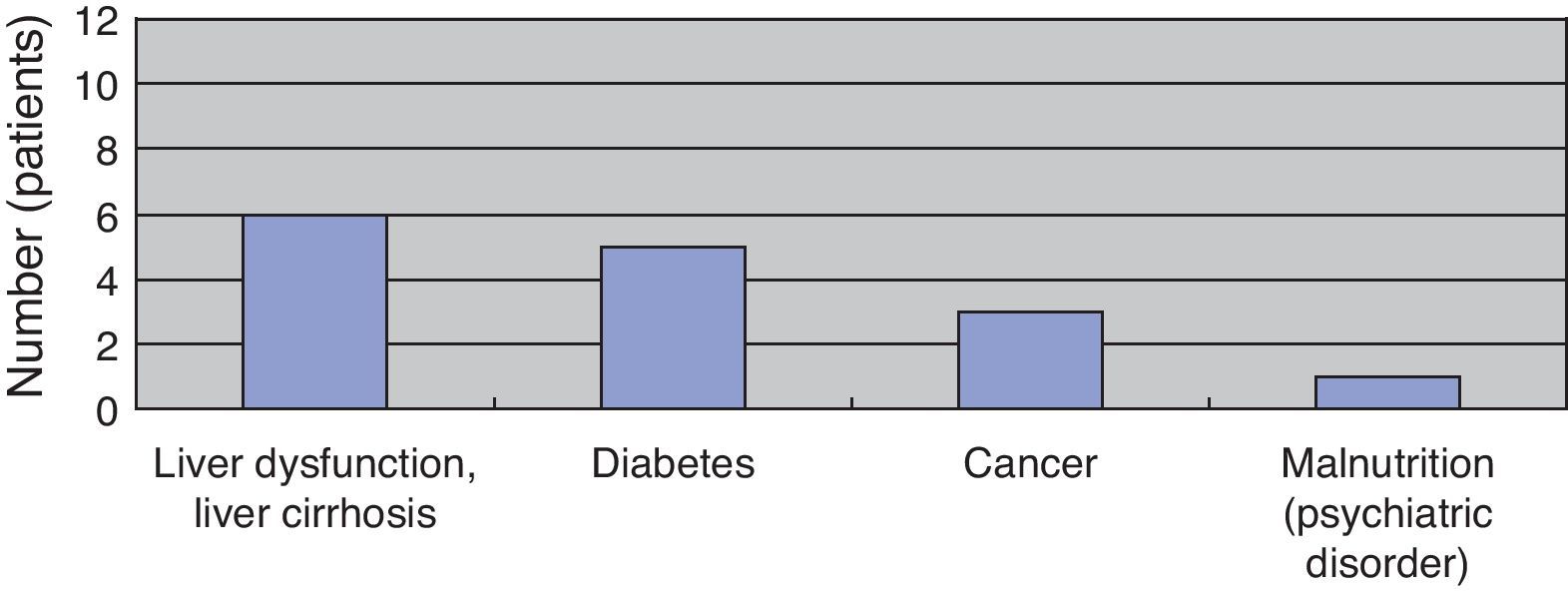
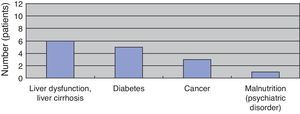
Results(Study 1) Patient characteristics and route of infectionThe study included eight men and four women (sex ratio, 2:1), all aged above 50 years (average age, 66 years). All patients had underlying diseases. Six, five, three, and one patient had liver dysfunction or liver cirrhosis, diabetes, cancer, and poor nutritional status due to mental disease, respectively. When two or more underlying diseases were present in one patient, they were overlapped and counted (Fig. 1). The time between fresh fish or shellfish consumption and disease onset ranged from several hours to two days for seven patients; this time was unknown for five patients.
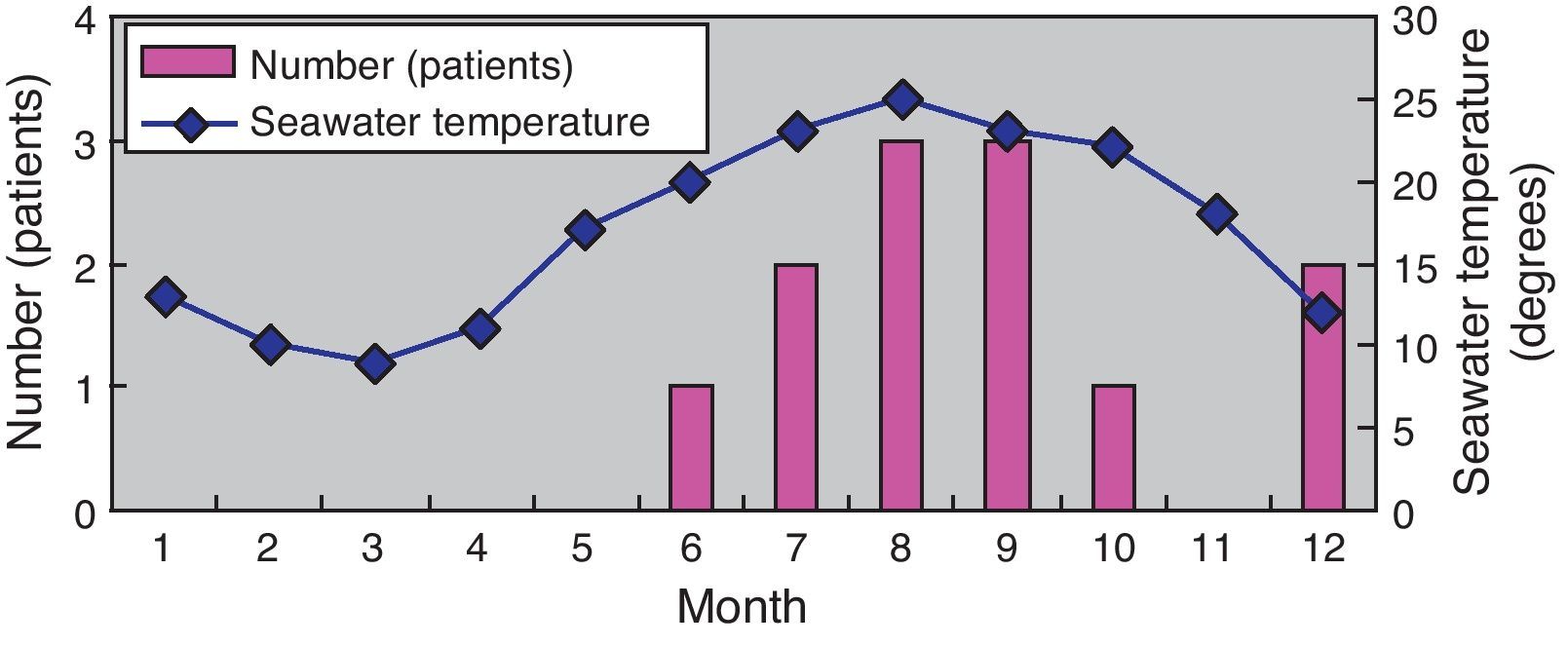
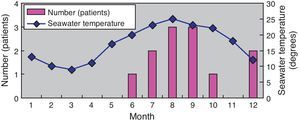
Next, the relationship between the month of symptom onset and seawater temperature in that month was investigated. Eight patients developed symptoms between June and September, whereas two, one, and one patient developed symptoms in December, May, and October, respectively. The seawater temperature along the coast of the Kyushu district is approximately 10°C from January to March, rising gradually by 10°C in April and May to exceed 20°C, where it remains until September before declining gradually. The graph created on the basis of these data indicated that the higher incidence of symptom development between June and September correlated with the increased seawater temperature during these months (Fig. 2).
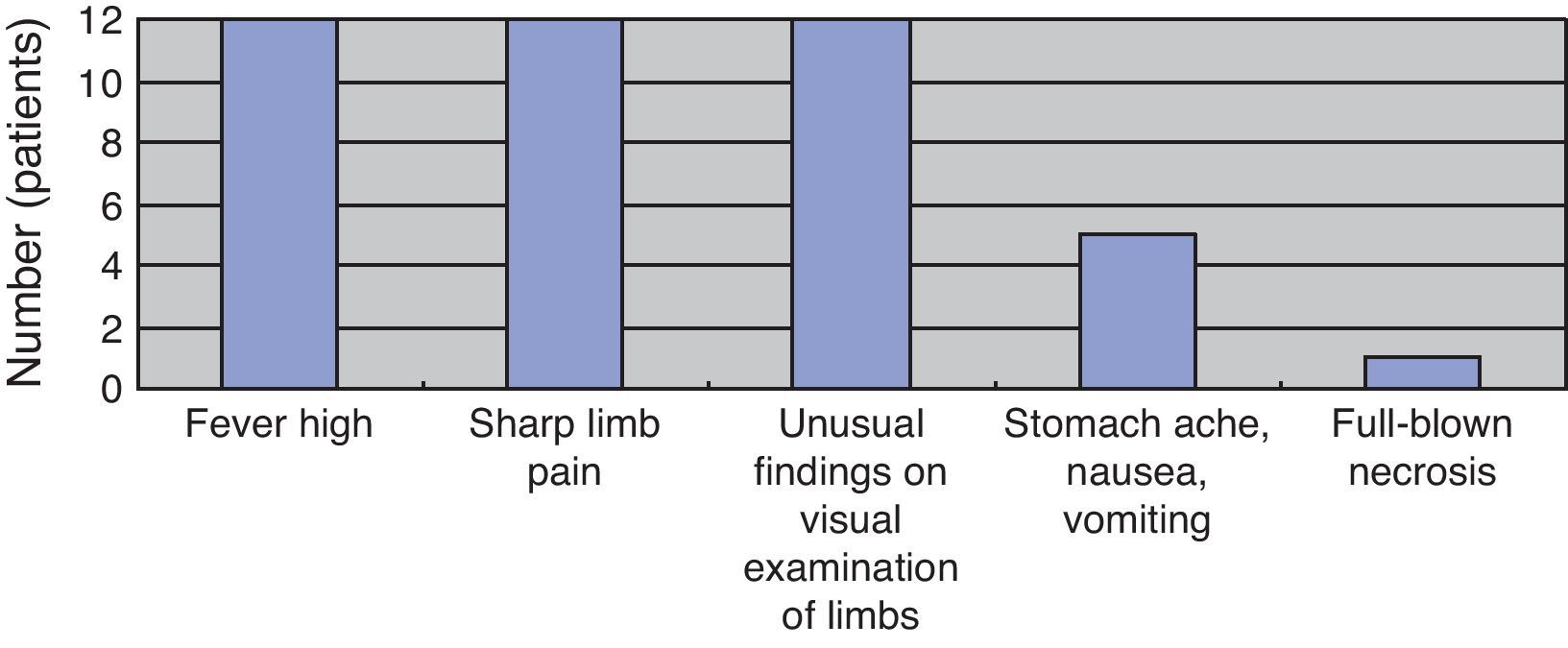
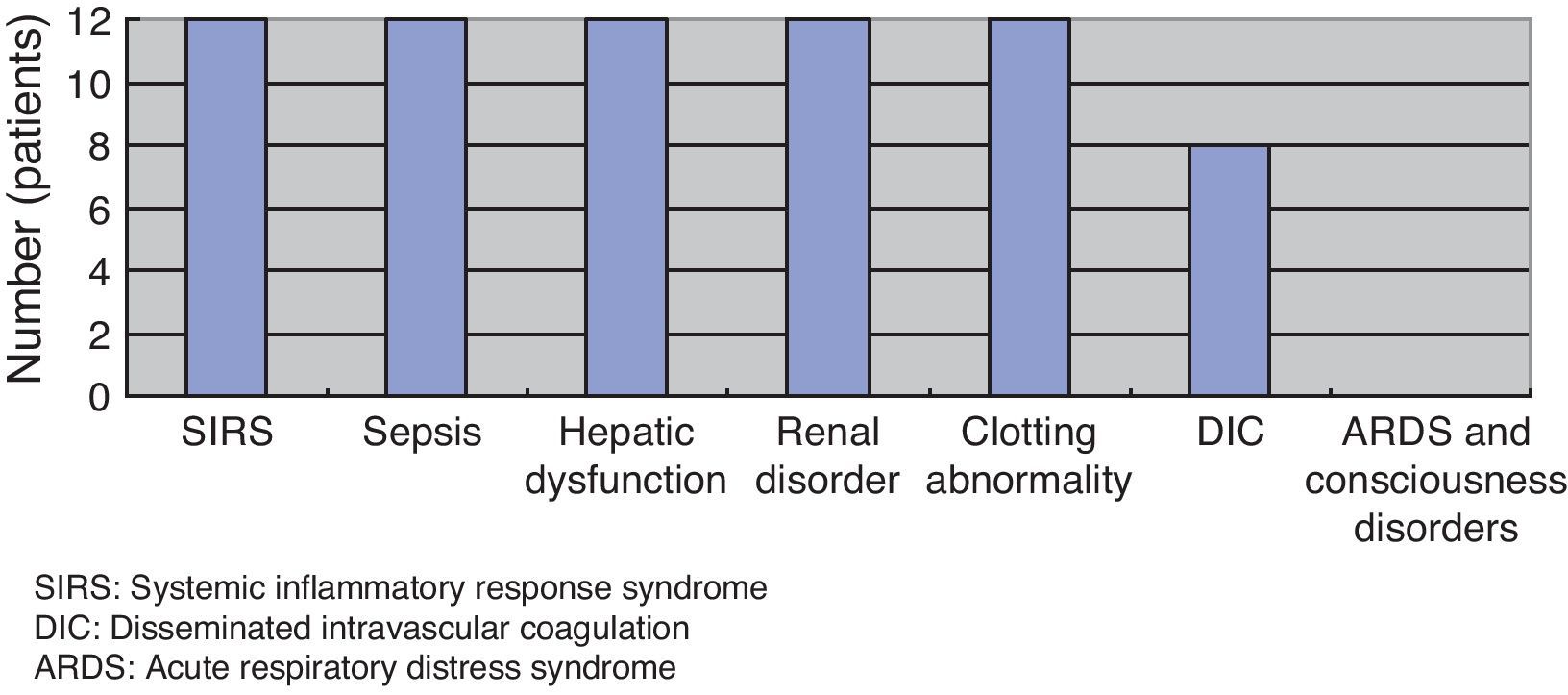
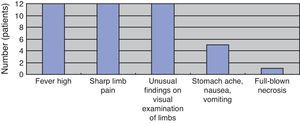
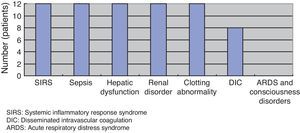
(Study 2) Physical and laboratory findingsAt the first medical examination, all patients exhibited fever and sharp pain in the limbs, which exhibited redness, swelling, purple spots, and/or blister formation on visual examination. Five patients exhibited stomachache, nausea, and vomiting. Only one patient exhibited full-blown necrosis (Fig. 3). As per laboratory findings, all patients had systemic inflammatory response syndrome (SIRS) and sepsis, hepatic dysfunction, renal disorders, and clotting abnormalities. Eight patients had disseminated intravascular coagulation (DIC). No patient had acute respiratory distress syndrome (ARDS) or consciousness disorders (Fig. 4).
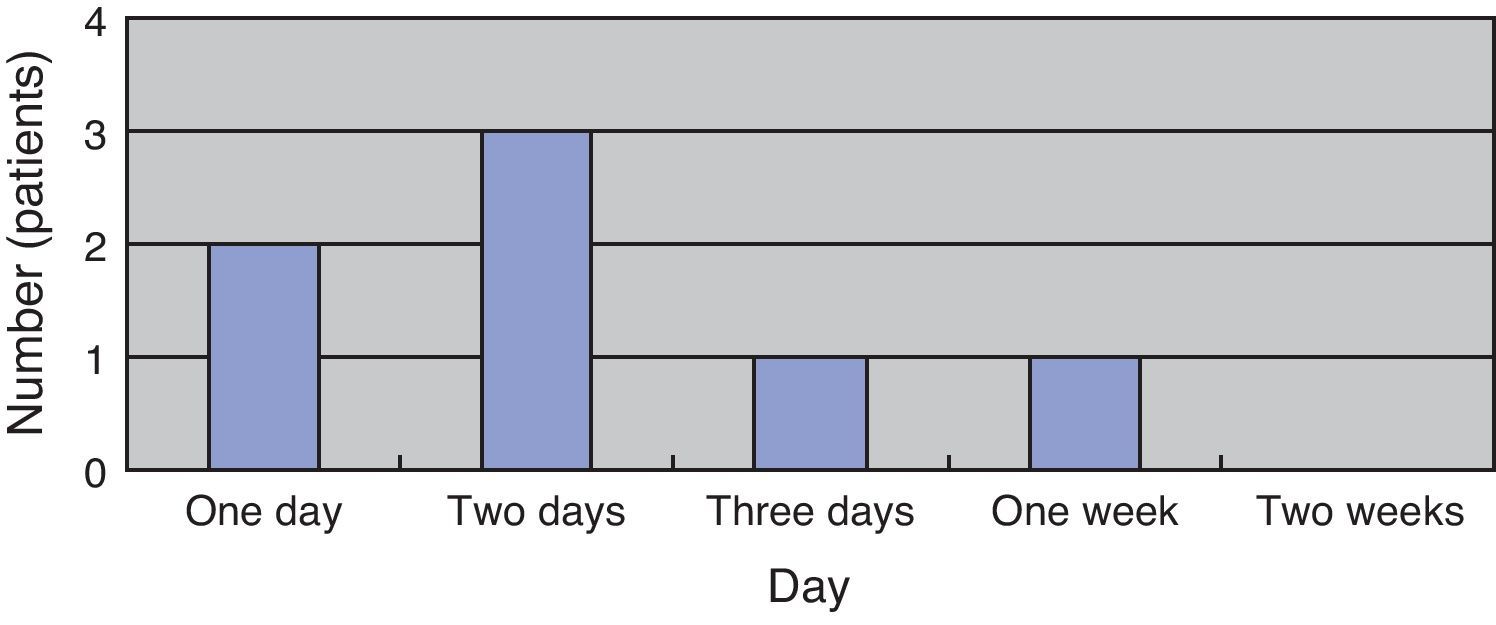
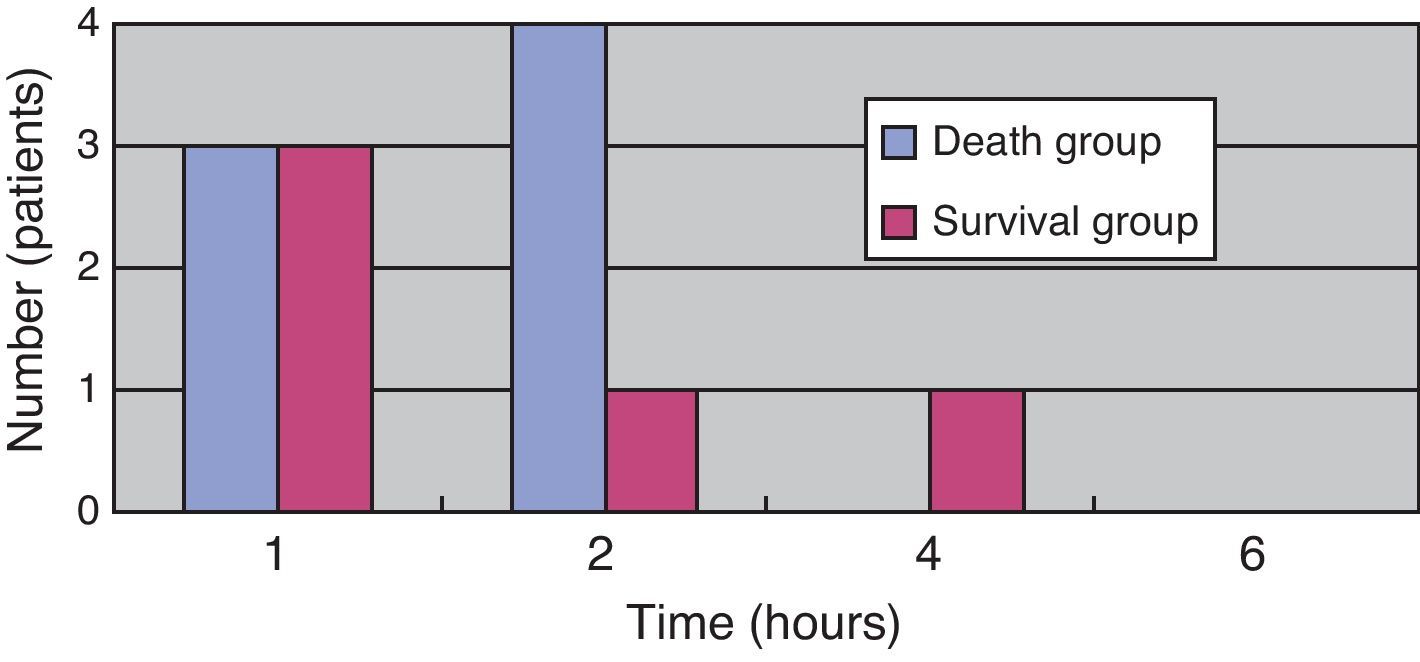
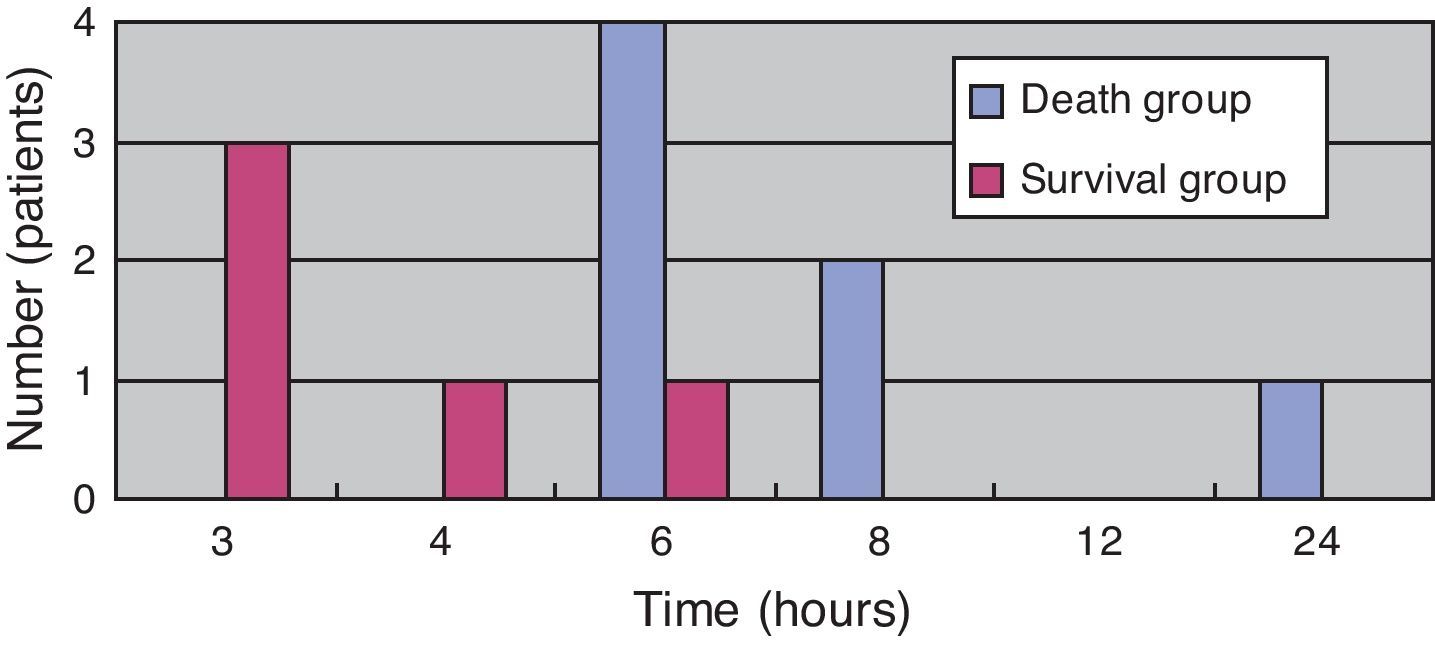
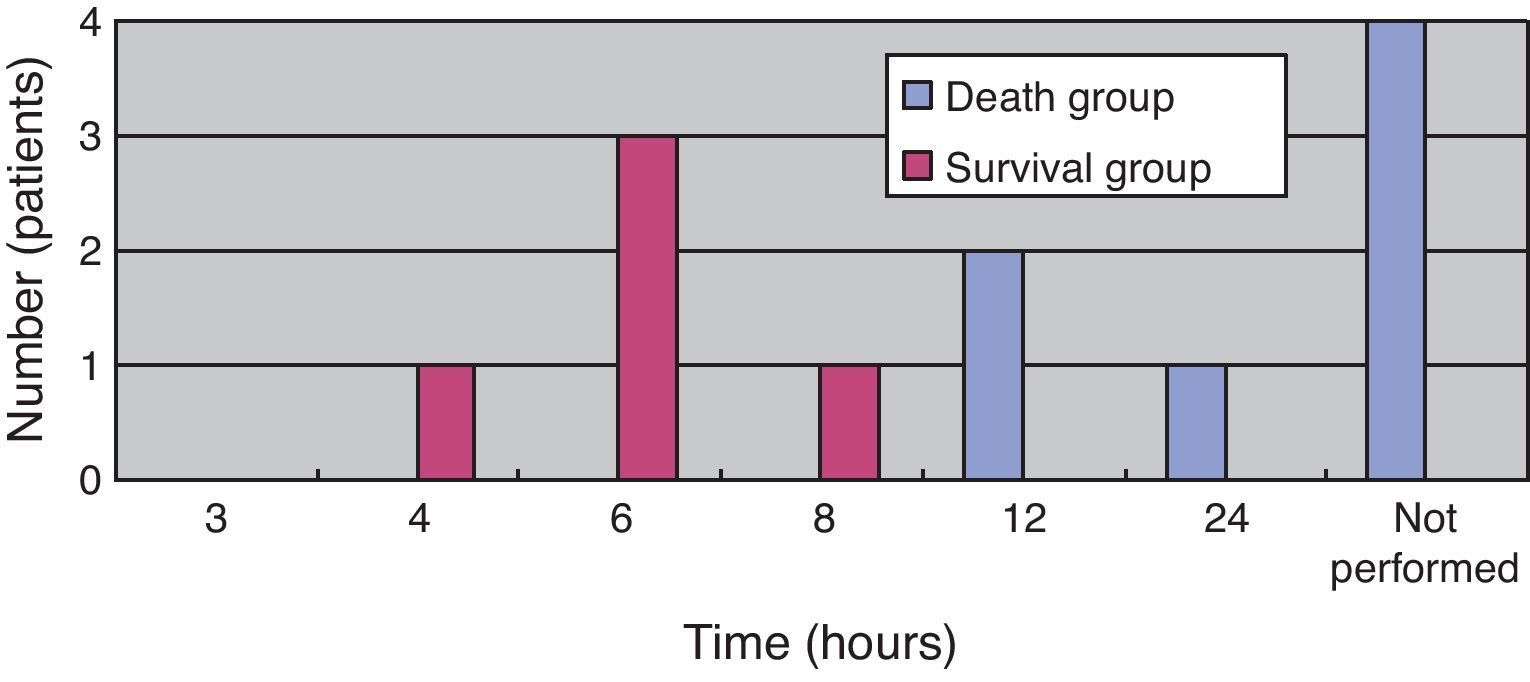
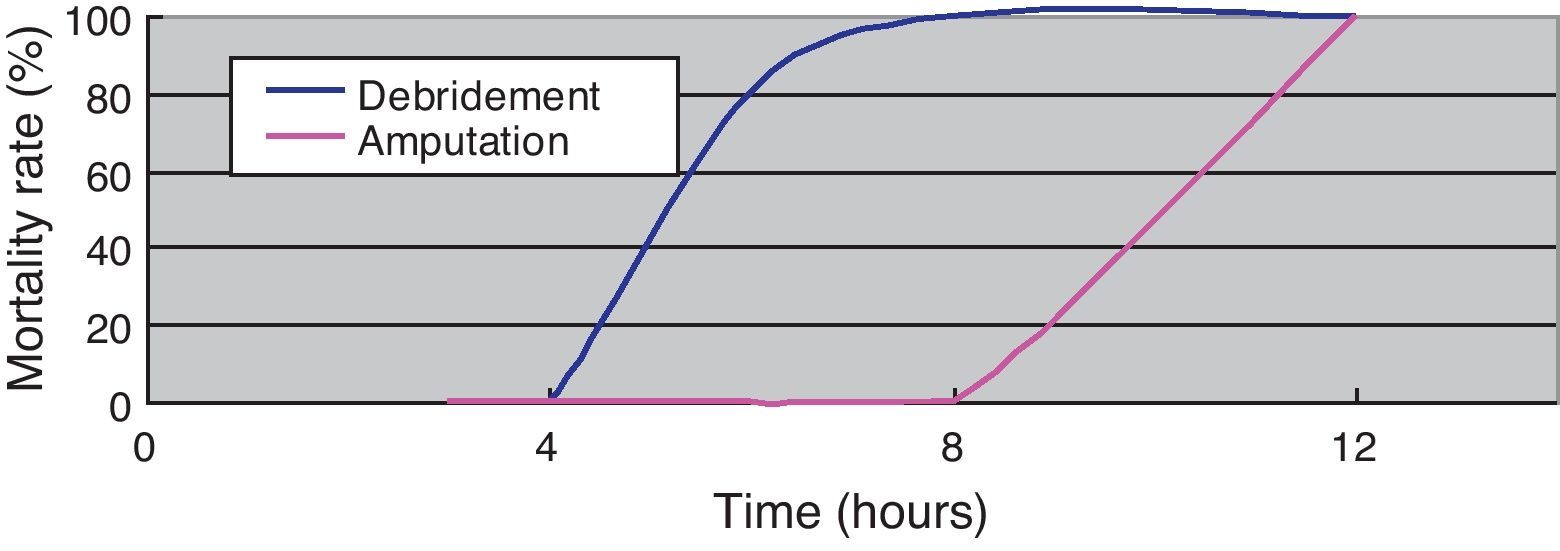
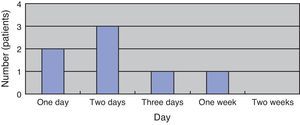
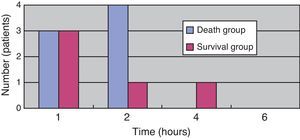
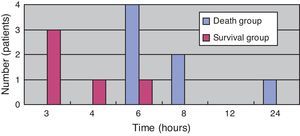
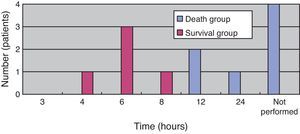
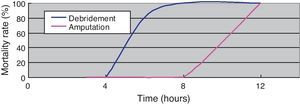
(Study 3) Treatment differences between the survival and death groupsThe mortality rate of the patients was 58.3% (7/12); 71% (5/7) patients died within two days (Fig. 5). There was no difference between the two groups in the time between symptom onset and antibiotic initiation, and the types and doses of antibiotics were similar for both groups (Fig. 6). No patient was treated only with antibiotics. Although all patients underwent debridement, it was performed earlier in the survival group than in the death group (Fig. 7). All patients in the survival group underwent amputation, which was performed early in many of these patients (Fig. 8). Two patients converted to amputation during debridement itself. Three patients underwent amputation 3h after debridement because of circulatory instability and abnormal leg skin color. Using the time of the first amputation as a reference, it was calculated that the survival group underwent amputation earlier than the death group, and less than half death group patients were eligible for amputation. Mortality increases if the time between disease onset and amputation exceeds 8h, and it was 100% in patients in whom amputation was delayed by 12h. Regarding debridement, mortality increases if the time exceeds 4h, and it was 100% in patients in whom debridement was delayed by 8h. The relationship between mortality and time from disease onset to debridement and amputation was plotted on a graph (Fig. 9).
DiscussionAlthough V. vulnificus multiplies in approximately 10min at 20°C, its multiplication is controlled below 15°C.3 Moreover, it is believed that approximately one million bacilli are required to cause infection via oral ingestion.4 For V. vulnificus infection to materialize, uncooked and V. vulnificus bacilli that grew at a seawater temperature of at least 20°C must have been consumed by the patients via raw fresh fish or shellfish that ingested this bacilli, which in turn condensed in the digestive organs of these fish. Age, disease status, and immune function influence V. vulnificus multiplication, which could explain why all patients in this study were >50 years old and had underlying diseases such as diabetes and hepatic dysfunction.5 Additionally, most patients were infected between June and September, during which seawater temperature is elevated. Fifty-eight percent patients (7/12) reported a history of consuming fresh fish and shellfish; this rate could be higher if the history of the remaining 42% patients (5/12) was known. The two patients who developed symptoms in December may have consumed raw oysters, which reportedly have higher contamination rates than those of other shellfish.5,6
V. vulnificus infection, although rare, typically manifests between June and October in patients with the following characteristics: 60 years old, underlying diseases such as liver dysfunction and diabetes, consume raw fish and shellfish. Because the time of treatment initiation is important, identifying potential cases of infection is critical. Although fever, sharp limb pain, and limb abnormalities were observed in all patients, necrosis of the extremities, a defining characteristic of V. vulnificus infection, was identified only in one patient. Necrosis is uncommonly observed at the first examination; this is the primary cause of delayed diagnosis and medical treatment. If no limb severities are observed at initial examination, cellulitis/phlegmon may be suspected. Because V. vulnificus multiplies rapidly and increases to one million in 2h in a blood vessel, a patient's condition can deteriorate rapidly.7 Although skin necrosis accompanied by a blood blister is observed after several hours, numerous bacilli will be present in this blister and treatment will no longer be effective.8,9
An unexpectedly high number of abdominal symptoms were observed in our study. If a healthy person is infected by this bacillus, he/she will have only stomachache and diarrhea as symptoms. Is it the same phenomenon? Liver and renal dysfunction may be caused by an underlying disease, but these conditions may be obstacles to patient recovery. Clotting abnormalities were detected in all patients, and 70% exhibited the diagnostic criteria of DIC. Conversely, no consciousness disorders or respiratory abnormalities were observed at the first medical examination.
The mortality rate in this study was 58.3% (7/12), in line with previous findings.10 Although the system of emergency centers and intensive care units in our country has substantially improved of late, this infection remains troubling because patients can die within two days, as in our study. Because it takes 48h to identify the bacillus by laboratory culture, medical treatment started after identifying the causative pathogen would be ineffective.
Regarding treatment options, there is no definitive cure, although there is no objection to early intervention with antibiotics and surgery.11,12 No significant differences in the type of antibiotic, administered dose, or the time of first administration were observed in our study groups. V. vulnificus is a Gram-negative bacillus, and because it is not a resistant bacterium, present-day broad-spectrum antibiotics are considered effective. However, because this bacillus rapidly multiplies, it is unclear whether antibiotic administration is sufficient to improve the condition of immune-suppressed patients. In fact, no patient was treated only with antibiotics in our study.
The survival group in our study underwent debridement and amputation earlier than the death group. Although patients in the death group were stable at the first medical examination, treatment was delayed because their family doctors initially diagnosed them with cellulitis/phlegmon and designed a corresponding treatment plan. However, after several hours, their general state and skin condition deteriorated, and debridement was performed confusedly. Four patients died without surgery because of unstable general health. Debridement at an early stage and, if medical treatment is resisted, enforcing amputation of the lower extremities should be performed immediately to increase chances of patient survival. According to our data, debridement and amputation should be performed within 4h and 8h, respectively. Mortality will increase if this window is missed because V. vulnificus multiplies rapidly; therefore, it will be impossible to eliminate the bacillus after this time.13,14 To permit timely surgical management, early suspicion and diagnosis of this fatal infection is important.
A flow chart from diagnosis to treatment was created from the data obtained in this study (Table 1). Diagnosis of V. vulnificus is first based on suspicion. As noted previously, V. vulnificus infection is commonly misdiagnosed as cellulitis/phlegmon because of the lack of standardized diagnostic criteria. Combining clinical history with a medical examination is helpful in predicting V. vulnificus infection. Various cultures, particularly in thiosulphate-citrate-bile salts-sucrose (TCBS) agar medium, and blood and fluid contents of blood blisters15 should be submitted while beginning preparations or standby for an urgent surgery. If V. vulnificus is detected in a TCBS agar medium culture, diagnosis is quick and accurate.16 If a Gram-negative banana-shaped organism is detected by Gram staining, the possibility of V. vulnificus infection is very high. Antibiotics should be initiated after submitting cultures. Any antibiotic that is effective against Gram-negative bacilli is expected to be effective against V. vulnificus. X-rays of the affected limb are necessary to rule out gas gangrene. Blood tests will determine clotting abnormalities, liver or renal disorders, and DIC depending on the case. If blister formation is observed on the skin, debridement should be initiated under general anesthesia. Although abnormalities such as low blood pressure and bleeding tendencies are related to poor prognosis, debridement should be performed within 4h to maximize patient survival. Furthermore, depending on intraprocedural observations, amputation of the lower extremities should be performed without delay. The patient's general condition usually stabilizes after removal of the affected tissue. If this does not happen, further amputation of the lower extremity (reamputation) should proceed promptly within 8h. If detection and treatment are not performed early in the course of infection, the mortality rate is very high. The presence of underlying diseases such as liver dysfunction and diabetes and the presence of symptoms such as warmth, sharp limb pain, and skin abnormalities are characteristic features and aid in diagnosis. If diagnosis and treatment are delayed, the effectiveness of antibiotics, debridement, and amputation decreases. It is very important for a clinician to recognize the characteristic epidemiology and clinical picture identified in this research and to perform quick diagnosis and treatment according to the flow chart presented in this paper.
Flow chart from diagnosis to treatment of Vibrio vulnificus.
| Strategy |
| When a patient in his 60s presents with sharp pain in his/her leg, the following clinical history should be examined: |
| Is he/she at least 60 years old? |
| Does he/she have hepatic dysfunction and diabetes as underlying conditions? |
| Did his/her symptoms first appear between June and October? |
| Does he/she have a history of consuming raw food? |
| Next, a medical examination should be performed. |
| Does he/she have fever? |
| Does he/she have sharp limb pain? |
| Are certain abnormalities observed on his/her skin? |
| Does he/she have stomachache, nausea, or vomiting? |
| Does he/she have systemic inflammatory response syndrome? |
| When these conditions are confirmed, V. vulnificus infection is strongly suspected. |
| Medical treatment is initiated according to the following flow chart. |
| 1: |
| Urgent surgery is planned while maintaining a dynamic circulatory state. |
| Usually, consciousness is maintained, and the respiratory state is satisfactory in many cases. |
| We should consider focusing on stabilizing circulation first. |
| General anesthesia is quickly induced. |
| 2: |
| Blood and the liquid contents of blood blisters are submitted for culture. |
| The detection of a banana-shaped Gram-negative bacillus by Gram's staining is indicative of V. vulnificus. |
| As laboratory culture will require 2 days, medical treatment should be initiated before the result is obtained. |
| TCBS agar medium cultures should also be prepared. |
| 3: |
| Sufficient antibiotic doses are prescribed for the patient as soon as possible. |
| Any antibiotic that is effective against Gram-negative bacilli can be used. |
| Although it is not a resistant bacterium, the speed of multiplication of this bacterium is extremely rapid. |
| 4: |
| When performing chest X-rays required for general anesthesia, an X-ray of the affected limb should also be performed to rule out gas gangrene. |
| Check for gas patterns in subcutaneous tissue. |
| 5: |
| Clotting abnormalities, hepatic dysfunction, and renal disorders are judged on the basis of the inspection data. |
| Many patients will meet the criteria for a DIC diagnosis. |
| 6: |
| First, debridement is performed under general anesthesia. |
| Although abnormalities such as low blood pressure and bleeding tendencies indicate a poor prognosis, debridement should be performed within 4h to maximize patient survival. |
| To stabilize the patient's general condition, do not admit the patients to ICU; the general condition will not improve unless the bacillus is eliminated. |
| Depending on intraprocedural findings, leg amputation should be performed without hesitation. |
| The patient's general condition should be stabilized after treatment. |
| 7: |
| When the general state is not stabilized by debridement and amputation, it is likely that multiplication of the bacillus has not ceased. |
| In this case, re-amputation should be performed within 8h. |
Because V. vulnificus infection is very rare and follows an aggressive course, it is thought that death may occur in patients even before diagnosis. This report clarifies the diagnostic criteria for V. vulnificus infection, thereby aiding in early diagnosis worldwide. The strategy of this article will be helpful to inexperienced physicians or surgeons who do not specialize in infectious diseases.
ConclusionThe mortality rate for V. vulnificus infection remains high.
However, infected patients have typical characteristics. Patient survival is increased if the infection is diagnosed, or at least suspected, at the first medical examination.
The effectiveness of debridement and lower extremity amputation decreases over time.
The characteristic epidemiology and clinical picture presented in this paper should be recognized, and the possibility of saving patient's lives should be maximized by performing early diagnosis and treatment according to the flow chart presented in this paper.
Conflict of interestThe authors have no conflict of interest to declare.